
By Dave Donohue
As a firefighter and emergency responder, understanding the basics of chemistry and how materials react, whether on fire, spilled on the street, or in contact with a person, can help you make tactical decisions, including response and decontamination actions based on predicted chemical behaviors and hazards. This article describes basic concepts related to the atom and periodic table and common properties and hazards of elements.
- In the Demo Lab: Teaching Basic Hazmat Concepts
- Hazmat: Initial Actions and Chemical Assessment
- A Guide to Chemical Profiling
- Hazmat Safety: Understanding the Behavior of Gases
The Atom
If we take anything and begin to break it down into its component parts, we will eventually have piles of material that cannot be divided up further by ordinary methods. These pure components are known as elements. Take water, for example—it is composed of two parts of hydrogen and one part of oxygen. If we run electricity through the water, it will divide into its component parts of hydrogen and oxygen, which we can capture and separate into different containers. Neither oxygen nor hydrogen can be broken down further and retain its characteristics. Those individual pieces of oxygen and hydrogen are atoms.
Atoms are made up of protons and electrons, and often have neutrons, as well. The center of the atom is known as the nucleus, and that is where protons, which have a positive electrical charge, and neutrons, which have a neutral charge, are found. Orbiting the nucleus are electrons, which have a negative charge. Both protons and neutrons have a weight of one atomic mass unit (AMU), while electrons have a weight of 1/1,836 AMU. While we think of the electrons as orbiting the nucleus in a wide circle, like planets circling stars, that is not accurate; the shape varies based on the number of electrons in orbit. But for now, a simple orbit will work. In future articles, we will discuss the orbit shells and how they impact reactivity and how elements bond to make everything.
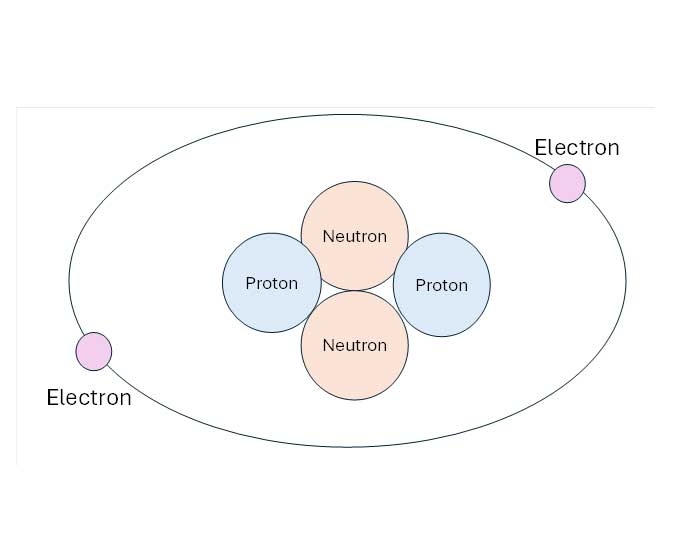
For our purposes, chemical reactions, including how reactive materials are, is based on how many, and where, electrons are found. When we look at the periodic table, elements with the same number of electrons in their outermost shell are grouped together and share similar properties. The atomic mass of an element is determined by adding up the number of protons and the average number of neutrons. The number of neutrons may vary, and the variations from the normal configuration are known as isotopes. The atomic number of the element is equal to the number of protons in the nucleus, which is how elements are identified. For example, chlorine may have 17 or 20 neutrons, but always has 17 protons and is placed as element 17 on the period table.
Characteristics of Atoms
- Atoms can be broken down into electrons, protons, and neutrons. Protons and neutrons can be further broken down into quarks.
- In any element, all the atoms have the same number of protons. Atoms of an element with a different number of neutrons are called isotopes.
- Atoms of different elements are different.
- Atoms of two or more elements can combine to form compounds.
- The atomic weight of an element reflects the weighted average of all its isotope forms.
- Atoms in a compound combine in a constant ratio.
Table 1. Characteristics of Atoms. Chen, L (2016)
| Particle | Location | Mass | Charge |
| Proton | Nucleus | ~1 AMU | +1 |
| Neutron | Nucleus | ~1 AMU | None |
| Electron | Orbitals | 1/1,836 AMU | -1 |
Table 2. Atomic particle characteristics. Gulch, I (2011)
The Periodic Table
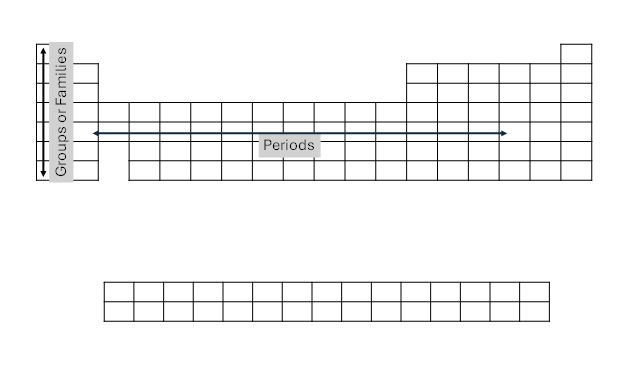
During the 1860s, several chemists were working on developing a means for classifying elements, with Dmitri Mendeleev publishing the basis of the periodic table as we know it today. Beginning with creating cards and laying them out linearly, Mendeleev noticed that there were repeating patterns in the chemical and physical properties. He then bunched the similar elements together in families or groups. As was later discovered, the similar properties of groups are related to the subatomic structure of the elements, primarily the number of electrons in the outermost orbital. Today we see these as vertical columns on the periodic table. While there were only 56 known elements, he was able to deduce that there were gaps in the table where future elements would be placed when they were discovered.
The elements on the periodic table can be further broken into three common categories: metals, nonmetals, and metalloids. To determine in which category an element can be found, begin by drawing a stair step line beginning below Boron (B). Also draw a line below Hydrogen (H). This line separates metals from nonmetals, with metals being to the left and nonmetals to the right of the line. The elements that touch the line are known as metalloids. Except for Mercury (Hg), metals are hard at room temperature, shiny, conduct electricity, can be drawn into a wire, and are malleable. Metals can be further broken down into the alkali metals, alkaline, earth metals, transition metals, and inner transition metals, Nonmetals may be found as solids, liquids, or gases at room temperature, including the natural gases of fluorine, chlorine, hydrogen, helium, nitrogen, and oxygen, and do not conduct electricity. Metalloids have intermediate properties, falling somewhere between metals and nonmetals.
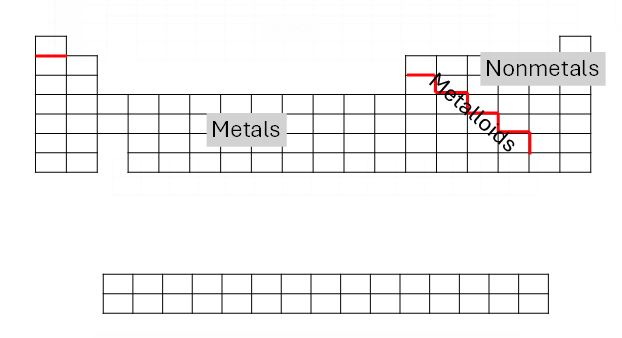
| Metals | Shiny, hard, malleable, ductile, high melting temperature, good conductor of electricity and heat, solids (exc. Mercury) |
| Metalloids | Characteristics of metals and nonmetals, some are semiconductors acting as both insulator and conductor of electricity |
| Nonmetals | Low density, low boiling point, poor conductors of electricity and heat, solids are brittle and low luster, may be solid, liquid or gas |
Table 3. Characteristics of metals, metalloids, and nonmetals
The first group or family, found to the left on the periodic table, are the alkali metals. These elements react violently with water, even humidified air; their water solutions are very caustic, requiring significant decontamination if contact with skin, and react with the halogens, meaning that halon fire extinguishers should not be used if there is a fire. They are frequently found naturally in salts. The next column represents alkaline earth metals. These are also reactive with water, but not to the extent of the alkali metals, and they can burn. The area between the second column and the metalloids are filled with the transitional metals. The transitional metals are shiny, hard, dense materials that are good conductors of electricity. The groups that are include boron, carbon, nitrogen, and oxygen are a mix of nonmetals, metalloids, and poor metals. The halogen family, headed by fluorine, are reactive gases and liquids (bromine) that often combine with metals. Finally, the noble gases, found at the far right of the periodic table, do not mix with other elements due to their electron configuration, which will be discussed in a future article.
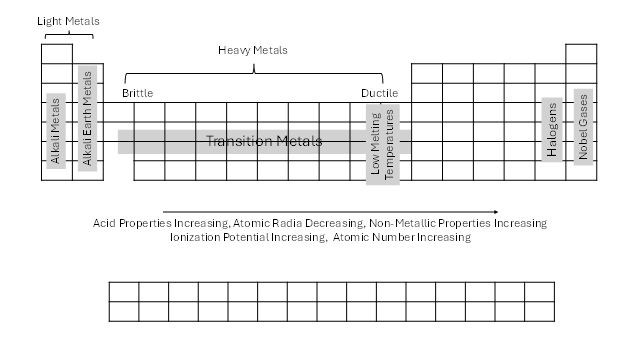
Figure 3. Periodic Trends
| Family | Common Properties | Examples |
| Alkali Metals | Highly reactive Rarely found in pure form React violently with water Produce caustic solutions with water | Lithium Sodium Potassium |
| Alkaline Earth Metals | Reactive Burn ‘ Gray with metallic brilliance React with acids to produce Hydrogen (flammable gas) Produce caustic solutions with water May ignite in air if heated | Magnesium Calcium |
| Transition Metals | Shiny Hard Dense Good conductors of heat and electricity Malleable and ductile | Copper Iron Nickel Gold Silver Mercury |
| Halogens | Reactive Most are gases Toxic Corrosive Oxidizers | Fluorine Chlorine |
| Noble Gases | Gases Inert Asphyxiant hazard | Helium Neon |
This article is intended to introduce responders to the periodic table and atomic structure to respond effectively and efficiently to emergency incidents. Future articles will examine how elements combine and the hazards associated with the mixtures and how emergency response is impacted. This article is intended to be street smart. Taking a chemistry course at a local college or the National Fire Academy will further your knowledge and provide you the tools to apply chemistry concepts during emergency response and during preincident planning.
Dave Donohue has more than 40 years of emergency response experience and has been a hazardous materials responders since 1982. He has served hazardous materials and fire department teams at the local, regional, and federal levels in several states and with the U. S. Coast Guard. He currently resides near Hagerstown, Maryland, and responds with the Community Volunteer Fire Company of District 12 in Fairplay. He can be reached at dkdonohue@aol.com.
Bibliography
Bevelaqua, A. and Norman, L. (2019). Hazardous Materials Chemistry, 3rd ed. Jones and Bartlett. Burlington, Massachusetts.
Biberdorf, K. (2021). It’s Elemental. The Hidden Chemistry in Everything. Park Row Books. Toronto, Canada.
Brunning, A, Cobb, C, et. al. (2022). The Chemistry Book. Big Ideas Simply Explained. Penguin Random House. New York, New York.
Chen, L. (2016). Barron’s Painless Chemistry, 2nd ed. Barron Publishing. Hauppauge, NY.
Cobb, C. and Fetterolf, M. (2010). The Joy of Chemistry. The Science of Familiar Things. Prometheus Books. Amherst, NY
Gulch, I. (2011). The Complete Idiots Guide to Chemistry, 3rd ed. Penguin Group. New York, New York.
Kernlon, M. and Mascetta, J. (2019). Barron’s Chemistry the Easy Way, 6th ed. Barron Publishing. New York, New York.
Lindley B. and Appleton, W. (2003). Street Smart Chemistry: Emergency Response Guide Featuring Weapons of Mass Destruction. E. I. du Pont de Neumors and Co. Wilmington, Delaware.
Meyer, E. (2005). Chemistry of Hazardous Materials, 4th ed. Pearson Prentice Hall. Upper Saddle River, New Jersey.
Russell, J. (2019). Elementary. The Periodic Table Explained. Michael O’Mara Books, Ltd. London, England.
Tro, N., ed. (2017). Know it All Chemistry. Wellfleet Press. New York, New York.

