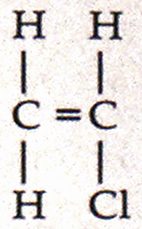
Chemical Data Notebook Series: Vinyl Chloride
FEATURES
HAZARDOUS MATERIALS
Vinyl chloride, a colorless flammable gas that is always shipped in its easily liquified state, is primarily used in the polymerization of polyvinyl chloride (PVC), the second highest volume plastic in the United States (polyethylene is the largest volume of plastic).
PROPERTIES AND APPEARANCE
Vinyl chloride is the monomer of PVC, which adds another hazard to this chemical’s already flammable nature. Monomers are shortchained molecules that have the unique capability of reacting with themselves to form polymers. This chemical reaction, called polymerization, liberates tremendous quantities of heat. If the polymerization gets out of control, or if vinyl chloride, also known as vinyl chloride monomer or VCM, is exposed to excessive energy in the form of heat or sunlight, a situation called runaway polymerization may occur. Under the proper conditions (if proper is the correct word), this reaction will transit to an explosion. There may be some debate as to whether the explosion is a BLEVE (boiling liquid-expanding vapor explosion) or a runaway polymerization explosion, but the results will be the same—disaster!
Vinyl chloride, whose chemical formula is C2H3CI, and whose structural formula is

is stable under “ordinary” conditions. The double bond, however, is reactive, and this is what makes VCM so valuable as a monomer. VCM has limited use as a raw material in the manufacture of other chemicals besides PVC, and can be used as a refrigerant and solvent.
Vinyl chloride has a very pleasant, sweet odor. It is just slightly less dense than water, having a specific gravity of 0.92, and only slightly soluble in water, which means that it will float on water. The molecular weight of vinyl chloride is 62.5, which makes the vapor density of the gas 2.16 (62.5 divided by 29, the average molecular weight of air). The implication of a vapor density of 2.16 is that any escaping gas will “hang together” and flow along the ground, seeking low spots. Since the gas is flammable, it may find an ignition source (VCM’s ignition temperature is 882°F) and flash back to the source of the vapor. VCM’s flammable range is from 3.6% to 33% in air.
IDENTIFICATION
Vinyl chloride’s UN (United Nations) designation is 1086, its STCC number (Standard Transportation Commodity Code) is 4905792. Its DOT (U.S. Department of Transportation) classification is flammable gas, and the IMO (International Maritime Organization) designation is 2.1. Its NFPA (National Fire Protection Association) 704 numbers are 2-4-1. Its CAS (Chemical Abstract Series) number is 75014.
Vinyl chloride has several synonyms in addition to vinyl chloride monomer and VCM. These include 1-chlorothylene, chloroethylene, monovinyl chloride, chloroethene, chlorethene, monochloroethylene, vinyl C monomer, VC, VCL, MVC, and ethylene monochloride.
HAZARDS Personal
VCM is not corrosive, but since it is shipped as a liquid (whose boiling point is 7.2°F), it will be very cold and may cause frostbite. Shortterm exposure to high concentrates of vinyl chloride (2.5% in air, or 25,000 ppm) can cause dizziness, nausea, headache, dulling of the senses, unconsciousness, and eventually death. There have been reports of people being exposed to 5,000 ppm over several hours without suffering any observable harmful effects. However, VCM is a carcinogen, and repeated exposures to these lower concentrations over long periods of time will be very dangerous.
The TLV (threshold limit value) for vinyl chloride is 5 ppm, while OSHA (Occupational Safety and Health Administration) permits only a 1 ppm TWA (time weighted average). Since VCM is so pleasant smelling, its health hazards may be easily overlooked.
PROTECTIVE CLOTHING
The emergency responder may protect himself from VCM by wearing protective clothing that is impervious to cold liquids. This will include rubber gloves and boots, and full faceshields with airtight eye protection. Positive pressure self-contained breathing apparatus is always recommended in a spill where one may be exposed to vapors. For low amounts of gas (less than 25 ppm) an approved organic vapor cartridge respirator (specific for VCM) will provide sufficient protection.
HANDLING
Even though VCM is stable to most chemicals, it is very reactive with copper and all copper alloys. Handling an incident involving VCM is one instance where brass tools should not be used. Since the gas is so flammable, you must eliminate all chances of sparks as ignition sources.
Precaution in an incident involving VCM would be the same as with any flammable gas that has been liquified. If liquid is leaking, large quantities of vapor will form as the material seeks to return to its natural state. Should the vapor ignite, the flame should not be extinguished until the flow of gas can be stopped.
Above all else, be aware that a BLEVE or runaway polymerization explosion may be imminent if the container has had direct flame impingement. Even without direct flame impingement, an explosion is possible if the container has been exposed to high heat (radiant heat). The containers should be cooled by unmanned monitors because of the threat of explosion.
VCM is stabilized for transportation (a material such as phenol is added to prevent premature polymerization); however, if this material is volatilized (driven out of the VCM by heat), the polymerization reaction may begin in a violent manner, resulting in an explosion.
Where unignited leaking vapors are the problem, the high vapor density of the gas alone will allow the vapors to travel great distances before dissipating, even if there is no appreciable wind. Any turbulence caused by the application of water, fog, or spray will aid in the early dissipation of vapors, lowering the chances of the vapors contacting an ignition source.
If fire does occur in a large VCM spill, treat it as any other flammable liquid. Since tremendous quantities of carbon monoxide and hydrogen chloride may be generated, respiratory protection is mandatory. All firefighters realize the hazards of carbon monoxide (CO), but might not be aware of the corrosiveness of hydrogen chloride (HC1). HC1 is very soluble in water, forming hydrochloric acid when it dissolves. You may easily remove the HC1 from the combustion gases downwind by “sweeping” the air with high pressure fog nozzles, but you must then contain the runoff water, which will be somewhat acidic.
Evacuation
If it is not possible to use water to disperse the gas and remove HC1 from the vapors, evacuation must be carried out downwind for as far as vapors may be detectable. This could be as much as two miles on a relatively calm day.
Glossary
BLEVE—Boiling liquid-expanding vapor explosion.
Double bond—Two covalent bonds between two atoms.
Easily llqulflable gas—A gas with a relatively high boiling point that can be reached easily by refrigerative.
Molecule—A chemical combination of two or more atoms.
Monomer—A short chain molecule capable of combining with itself to form a polymer.
NFPA 704—National Fire Protection Association’s standard for hazard warning for stationary containers.
Polymer—A very long chain molecule formed by hundreds and thousands of monomer molecules combining with themselves.
Polymerization—The chemical reaction in which monomers combine with themselves to form a polymer.
ppm—Parts per million (of air).
Runaway polymerization—The almost instantaneous conversion of monomers to polymers with a great release of heat energy. Under the “right” conditions, this is an explosion.
Specific gravity—The weight of a solid or liquid as compared to the weight of an equal volume of water.
Vapor density—The ratio of the molecular weight of a vapor or gas to the average molecular weight of air (29).
Since VCM is shipped in railcars and tank trucks (and perhaps in smaller containers) evacuation and withdrawal distances will also depend on the amount of product involved in the incident. Evacuation distances of one-half mile to a full mile are recommended where tank trucks and railcars are involved.
In addition to the harmful effects of VCM vapor inhalation, evacuation may be necessary because of the explosion hazard. All ignition sources must be removed, and any liquid must be prevented from entering waterways.
Containment
If entry into a waterway occurs, notify users of downstream water and all agencies charged with protection of the environment.
If the stream is small, containment dikes or dams may be built to hold or divert the contaminated water to another holding area. However, care must be given to maintenance of the structure to prevent unexpected collapse and release of large quantities of contaminated water.
Where the amount of liquid VCM entering a stream has been small, absorbing agents, such as activated charcoal and peat moss, may be used to capture dissolved contaminant. Be advised that as spilled liquid enters waterways, the water will be significantly warmer than the VCM, causing evaporation to accelerate.
Prevention of contamination in sewers is much simpler because the openings of catch basins and other entryways into the sewer system are easily dammed by soil, sand, or other sorbents.
Wherever liquid vinyl chloride monomer is released on land, the material may be contained by building containment dikes of soil, sand, or other sorbents. All contaminated soil will have to be removed and taken to a qualified landfill.
If containment of the spill is called for in a particular incident, one must keep uppermost in mind the fact that an easily liquifiable flammable gas is involved. During the incident, emergency responders can become so engrossed in containment techniques that they forget the principal hazard of the spilled material. Remember that as long as the liquid remains exposed to the atmosphere at temperatures above its boiling point, flammable vapors are being produced—vapors that are significantly heavier than air and that have the capability of moving along the ground great distances before contacting an ignition source.
Again, in all cases of release of VCM, the proper authorities must be notified. If containment is successful, the manufacturers, shippers, and consignees of the VCM should be contacted to see if they can help keep the production of vapors down. These people will also prove helpful in recovering any remaining liquid by transferring it into undamaged containers.
Ditches and trenches may be dug at the site to lead the liquid to a safer place of containment, perhaps a deep pit that can be covered to prevent vapor release.
After the liquid is contained, it must be pumped into secure pressure containers as soon as possible to lessen the dangers of explosion and/or contamination.
If the VCM container is ruptured, the liquid will be forced out under some pressure. If the liquid can be contained or caught in a container of some sort, a portable pump may be used to recirculate the liquid back into the leaking container. This may not be possible in warm weather, since vapor pressure inside the tank may prevent liquid from reentering. If this technique is attempted, remember that you are dealing with a flammable gas. All ignition sources must be eliminated, or tragedy will follow. Even the pump used must be explosion-proof to prevent it from being an ignition source.
First aid for those exposed to the gas include fresh air, artificial respiration, and immediate medical attention. Where contact has been made with the liquid, flushing with water for 15 minutes is recommended, especially for the eyes. All contaminated clothing should be removed, and skin should be washed with water and mild soap. All unprotected personnel should remain upwind and out of contact with the liquid.
SUMMARY
Knowing the major use of vinyl chloride, emergency responders who have PVC polymerizing plants in their response districts should prepare themselves for an eventual spill. By contacting the railroads and trucking companies, responders can determine the routes by which VCM is shipped through their districts.
Since VCM releases can accumulate in basements and other confined spaces, care must be taken to ventilate all such low-lying or confined areas. An otherwise successful mitigation of a VCM incident might be marred by an explosion or asphyxiation occurring because someone failed to look for it in likely places.

