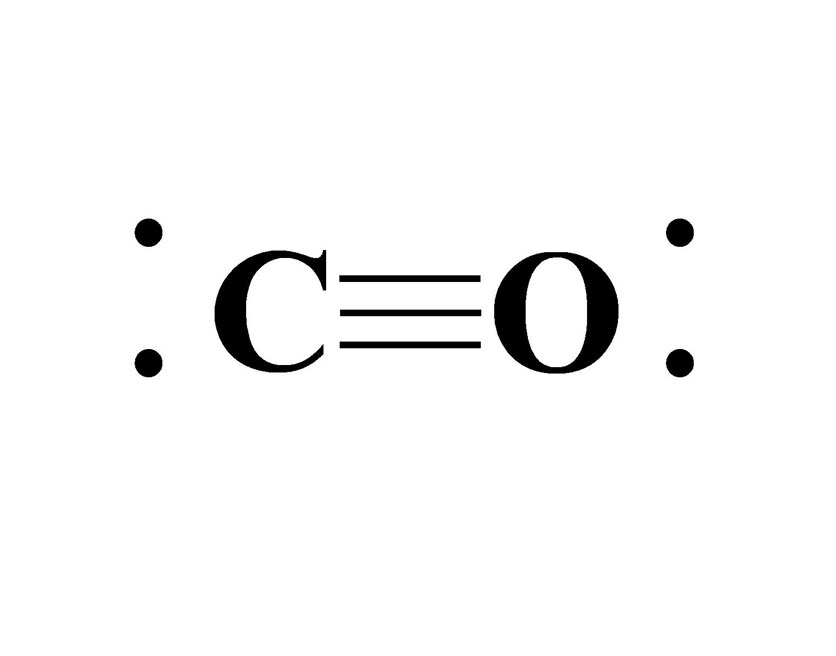
By Robert Bohrer
A Case Study
Your fire station gets a call for an “unresponsive person.” Both rescue (medic unit) and fire engine get dispatched to this call. While en route to the address, dispatch updates the responding units with information gathered from the caller. The information from dispatch is that the patient is a male in his 50s who is not responding, and the caller is unsure if he is breathing or not. Per the caller, the patient will be located in the driver seat of a vehicle inside of a garage.
Upon arrival of the fire rescue units, they observe multiple three-story condo/town home buildings that are very close to each other with the first floor being the garage. The caller is standing in an alleyway between two of the buildings, waving at the fire rescue personnel. The rescue truck parks between the two buildings in the alley just a bit past an open garage door where the patient is located. As the fire medics get out of the rescue truck, the neighbor (who called 911) frantically runs over to them, saying: “My neighbor is in the car over there, he isn’t responding and I don’t think he is breathing. I know he has some medical problems, heart issues being one of them, so maybe he had a heart attack. I shook him to try and wake him, but he didn’t, and I didn’t feel a pulse, but I might have been too nervous to actually feel it.”
RELATED
- Construction Concerns: Carbon Monoxide
- Ontario Firefighters Launch Carbon Monoxide Safety Video
- THE DANGERS OF CARBON MONOXIDE AT THE “ROUTINE FIRE”
FirefighterNation: Near Miss: Post-Fire Metering for Carbon Monoxide
The fire medics unload the stretcher from the rescue truck and make their way over to the garage. As they approach the building, the small carbon monoxide (CO) detector attached to the EKG monitor on the stretcher begins to alert and beep. All the fire rescue personnel on-scene immediately stop and back away from the garage where the patient is located. Just then, the neighbor who called 911 leans against the rescue truck and begins vomiting, which causes her to fall to the ground.
The company officer immediately has the rescue crew put the vomiting neighbor in the rescue truck for medical treatment and then calls for a hazmat response. While the rescue crew assesses the neighbor, they find she is complaining of a severe headache. The company officer starts to have his engine company crew put on their bunker gear and self-contained breathing apparatus (SCBA). Before the company officer gives the order for his engine crew to make entry to check the patient inside of the garage, he asks the neighbor: “What made you go check on your neighbor and how did you know he was in the garage”? She responds: “I smelled exhaust in my apartment, so I went outside to see where it was coming from and realized it was very strong smelling outside of my neighbor’s garage. Plus, I thought I could hear a car running inside, so I went through the front door, because he had given me a key. I made my way into the garage and there he was in the front seat, and it looked like he was sleeping, but the whole garage was filled with exhaust. That’s when I opened the garage door, checked on him, and then called 911”.
The company officer gives the order to his engine crew to go on air and make entry to check the patient in the garage. The engine crew makes contact with the patient and confirms that the patient is deceased. The company officer withdraws his crew and gives the order to start checking the neighboring apartments for other people that might still be inside and begin evacuation procedures of the building. While the evacuation of the building is under way, a battalion chief and the hazmat team arrive on-scene.
When we think of an asphyxiant there are two types “simple” and “systemic”. Simple asphyxiants are inert gases or vapors that displace oxygen (O2), creating an oxygen-deficient environment that cannot sustain life. A few examples of simple asphyxiants are carbon dioxide (CO2), nitrogen (N), helium (He), methane (CH4), propane (C3H8), and argon (Ar). If sufficient amounts of these inert gases are released into an enclosed environment, it will begin pushing the oxygen out and replacing it with the inert gas. Thus, exposure to this type of environment leads to hypoxia (lack of oxygen) for the victim, which could progress to unconsciousness or death depending on how oxygen-deficient the environment is.
However, CO is not a simple asphyxiant, but rather a systemic asphyxiant, because it alters or prevents the body’s uptake and absorption of oxygen at the cellular level. Another systemic asphyxiant that is sometimes present with CO, especially in structure fire smoke, is hydrogen cyanide. When these two toxic gases are present, they are called the “toxic twins.” CO has a 210-250 times more affinity than O2 does to hemoglobin, making it extremely toxic. Although CO being an inhalation toxin at the cellular level, it does not damage the lung tissue itself. Therefore, if a victim is poisoned by CO and receives early medical treatment to remove the CO off the hemoglobin and replace it with O2, then the prognosis is usually positive without having to worry about any adverse effects.
So why is CO called a “silent killer”? First, it is odorless and colorless. This is the main problem that is contended with when responding to certain emergency calls, however that is not the only hazard with this gas. CO is flammable as well, so the complacent approach of “just go in real quick with the CO monitor” or “you only need your SCBA on” is something that may get responding crews hurt or killed.
According to the CDC, every year at least 430 people die from unintentional CO poisoning, yet these deaths are in no way linked to the victims being exposed to fires. About 6,000 people die each year total from CO poisoning, and approximately 50,000 people visit the ER.
Why are there such high numbers of injuries and/or deaths from CO poisonings, especially with all the information and public service announcements about this gas? It could be lack of education on the subject, or it could be that it never crosses anyone’s mind that they could potentially be in harm’s way with CO. The speculation of how the message about CO doesn’t resonate with some people would probably keep you up at night. That is why as public servants we need to educate the community continuously on the dangers of CO. We must remember that CO not only affects humans, but animals as well. Animals and children will feel the effects of CO poisoning earlier, and more readily than an otherwise healthy adult (depending on the amount and duration of exposure).
Another part of protecting the community through education is performing surveillance on how many CO calls your area responds to. If there is an increase in CO emergency calls (whether they are detector activations or unintentional/intentional exposures) then this could potentially direct your agency with conducting more CO awareness seminars, handing out more printed material about CO, or reaching out to the news for a public service announcements (PSA) about CO. The list goes on, but at least this will hopefully allow a dialogue to open up about the importance of educating and protecting the community from CO poisoning, and how your department is taking in active role in getting the word out.
How Carbon Monoxide Is Formed
CO forms due to incomplete combustion of hydrocarbon fuels that are burning. Complete combustion occurs as long as there is a good amount of O2 in the air while the fuel is burning. When this process is happening, carbon and hydrogen atoms that are contained in the hydrocarbon react with the O2 in the air as an exothermic (energy release) reaction. The result of this exothermic reaction is the formation of carbon dioxide (CO2), water vapor, and whatever amount of energy produced. With incomplete combustion, there is a lower amount of O2 in the air to help support the complete combustion of the hydrocarbon fuel. The carbon and oxygen atoms form a triple bond together to balance both atoms out. A triple bond that is formed between these two atoms is known as a covalent bond (picture 1, above), which is when atoms share their electrons with each other. A side note to keep in mind about chemicals that form double and triple bonds is that it makes the chemical very unstable. This instability can come in the form of toxicity, flammability, or volatile reactions such as polymerization. It’s due mainly in part to the amount of energy the chemicals (that are double or triple bonded) can potentially release when or if they are separated. This is how CO is formed as a byproduct of the incomplete combustion process and what makes it a very unstable chemical. Water vapor is still produced and so is carbon during incomplete combustion, but the formation of CO is the toxic result.
Carbon Monoxide Chemical Information
UN number: 1016
NFPA 704 placard: Health 3 – Serious, Flammability 4 – Extreme, Instability 0 – Minimal, Special Hazards – NA
Molecular formula: CO
Molecular weight: 28.01 (slightly lighter than air)
IDLH: 1,200 ppm (very deadly)
Vapor density: 0.968 (Air = 1)
Boiling point: -313°F (-191.5°C)
Melting point: -337°F (-205.02°C)
Lower explosive limit (LEL): 12 percent
Upper explosive limit (UEL): 74 percent
Soluble in: Water
Classification: Hazmat Class 2 – Gases, Div. 2.1: Flammable gases, Div. 2.3: Toxic gases
CO is extremely flammable, highly toxic, and is the top cause of inhalation death. CO poisoning works by binding to the hemoglobin in the victim’s body, which prevents O2 molecules from attaching during normal respiration. CO has an affinity of more than 210-250 times greater than O2 when it comes to binding with the hemoglobin. This can be very problematic to anyone that is exposed to CO, because it suffocates the victim at a cellular level due to hypoxia (lack of oxygen). The CO will starve the body of O2, because it pushes the O2 off the hemoglobin and replaces it with CO molecules, creating a condition called carboxyhemoglobin (COHb). With this formation of COHb it causes a decrease in the O2 carrying capabilities of the blood, which impairs the release of the O2 from the hemoglobin to the tissues.
| Percents of CO Levels in the Body | Signs and Symptoms |
| 20 percent | Headache • Nausea • Vomiting • Dizziness • Blurred vision • Loss of manual dexterity |
| 30 percent | Altered mental status • Chest pain • Lethargy • Tachypnea, Tachycardia • Rhabdomyolysis • SyncopeImpaired judgement • Loss of coordination • Decreased motor abilities • Temporary blindness • Hearing loss |
| 40-60 percent | Hypotension • Cardiac arrest • Respiratory arrest • Dysrhythmias • Seizures • Convulsions • Coma |
| >60 percent | Death |
Carbon Monoxide Response
At some point in a first responder’ss career, they will respond to a call involving CO. These particular calls don’t always end up being an emergency with deceased victims or very ill patients from a CO poisoning. This call could be just someone’s in-home CO detector activating because of a battery issue or a cross-sensitivity activation due to another chemical.
Speaking of an in-home detector having chemical cross-sensitivity, a very common CO detector alarm call is from hydrogen gas released from golf cart battery charging or just battery charging in general. Although hydrogen is a completely different gas than CO, it sometimes causes the in-home CO detectors to activate. I have responded hundreds of times to this type of CO alarm call (on top of calls with actual CO present). Every time the full precautions were always taken as if there was really CO in the residence or business. After monitoring the building with a 5- gas monitor, we were usually able to narrow the problem down to a golf cart battery that was overcharged causing a release of hydrogen gas, which in turn activated the CO detector on the wall.
Another issue that can arise with CO is the potential for the gas to explode. The buildup of the gas can enter void spaces or completely fill up a building (that might be vacant or evacuated) because of an earlier fire, faulty appliance, or vehicle exhaust. A smoldering fire inside of a building or underground (sewers, basements, vaults, etc.) is creating CO, and this can cause the gas to build up to an explosive level. If there is enough CO in the area in its explosive range (12-74 percent), then something as simple as a light switch being flipped on or a refrigerator compressor clicking on could cause ignition. This should further drive the point home of taking every precaution when responding to these types of calls, including wearing the proper PPE and creating an evacuation zone for civilians.
First and foremost, if you are a non-hazmat company, request a hazmat team immediately. Don’t think by requesting a hazmat team that it is undermining you and your crew. A hazmat team has an assortment of monitoring devices and extra equipment to help mitigate the situation. A hazmat team response is supplementing the first-arriving non-hazmat companies that are already on scene.

Before performing CO monitoring with either a 4- or 5-gas monitor, make sure to perform a “fresh air” calibration away from the building in question or any running vehicles (picture 2). The goal for this calibration (before you go into a toxic environment) is to allow the gas monitor to warm up and have a clean background on the sensors, which will help the first responders truly see if there is any kind of toxin in the air when they make entry.
Some important aspects of using a gas monitor is establishing the hot zone and to follow up on whether the mitigation of the problem is working or not (i.e. ventilating the building). After the “fresh air” calibration is properly performed you will begin to approach the area in question. Once the gas monitor alerts you to the presence of CO, you have now established your hot zone. Remember, you must know your limitations of the gas monitor you are using, because if you take that monitor into the hot zone and saturate the sensor with the toxin, you could potentially damage the monitor. If the sensor is damaged, then this puts you and everyone at risk, because the tool you were using to look at atmospheric toxin and flammability levels is now inaccurate.
On a side note to saturating a gas monitor’s sensor, please avoid sticking the end of the monitor into an exhaust pipe on your apparatus during your morning check. By doing that you are damaging your monitor. If you need the device later during a real call, you may not have accurate readings. If the sensors in the monitor are saturated and damaged, then the monitor must be taken out of service. If your agency has someone who is certified to work on the monitors, then they could simply change out a bad sensor for a new one. If your agency doesn’t have anyone certified to work on the monitors, then the monitors will have to be sent back to the manufacturer for repair. Please, don’t try and change any sensors or fix a monitor if you are not qualified to do so. You could potentially be taking on liability and voiding any manufacturer warranties on the monitor(s).
Another point to emphasize is that your gas monitor can alert due to cross-sensitivity of other chemicals other than CO. Depending on the brand of gas monitor your agency uses, the manufacturer should include a booklet that has various cross-sensitivity conversions for chemicals that your monitor doesn’t necessarily have sensors installed for.
Carbon Monoxide Response with Viable Victims
What should be the proper actions of first responders arriving at a CO call with a viable victims? First and foremost, if you are a non-hazmat company, request a hazmat team immediately. Don proper PPE before making entry, which is bunker gear and SCBA. If a line-of -sight rescue of viable victims is present, then go ahead and perform that task. Once the victims are removed from toxic atmosphere and moved to a safe distance, decontamination procedures can be performed. The decontamination of a CO victim can be as simple as removing the clothing. Remember removing a victim’s clothing can remove 80-90 percent of the hazardous contaminate. If a victim has been exposed to CO for a prolonged time with an extremely high level of the gas in the environment, then decontamination will need to be performed, because the CO can absorb through the skin. A good indicator of a severe exposure with dermal absorption is the skin may be a cherry red color. The victim’s clothes can be left outside to be aired out, or they can be placed in a bag with a tie and sent with the victim to the hospital. Once any victims are removed from the hazardous area, then atmospheric monitoring and mitigation procedures can be started.
Warning Signs When Approaching a Scene and Making Entry into a Building
- Patient is unconscious and unresponsive (Note: Children are more susceptible to early effects of CO poisoning)
- Incapacitated or deceased animals (i.e. dogs, cats, birds, etc.)
- In-home CO detector beeping or light activation
- Suicide notes and posted notes warning rescuers
- Bystanders or neighbors complaining of CO poisoning symptoms (Note: This frequently occurs in attached apartment or town home-type buildings)
Always maintain scene control and rescuer safety when responding to these types of emergencies, especially when called out for welfare checks (i.e. do not allow anyone in without proper PPE on) . You must wear PPE, including respiratory protection, and this needs to be donned prior to entering a scene where CO is present.
The highest level of respiratory protection is wearing SCBA and making sure personnel are properly trained and OSHA 29 CFR 1910.134 compliant.
If the patient is declared deceased where they are found, then leave the body where it is. It becomes a crime scene, so maintain the integrity of the scene for the law enforcement investigation.
Another consideration for rescuers regarding CO is the fact that it is slightly lighter than air. If you are ventilating the structure, the vapor may not leave as quickly as you would like. The mitigation of CO from a building may take some time depending on the levels the gas monitor is indicating. CO is a very sneaky gas that likes to find other areas of a building that are a good distance from the actual source. This holds true in multi-occupancy buildings (i.e. large apartments, condos, row homes, offices, basements, etc.). The factor that may allow CO to travel throughout a building is through a common attic, openings in the wall from pipes or damage, or even firefighting operations (e.g. ventilation, fire streams, forcible entry, etc.).
It is imperative that an entire building should have atmospheric monitoring with a secondary search performed after firefighting operations are completed. CO can be found at the farthest point of a large multi-occupancy building from the initial source, whether it was a fire, vehicle running inside, or a faulty appliance. Much like smoke, CO will travel the path of least resistance. As CO begins its journey and travels away from the source, it may encounter a building’s ceiling or a wall and begin to have a mushroom effect (just like smoke). Also, as the CO travels away from the source it will begin to cool. This causes it to sink and stay in voids between floors or hang around in other habitable areas of the building (or attached buildings).
There is research published by the Journal of the American Medical Association (JAMA) that tested to see if CO diffused through gypsum drywall. The findings showed that CO did indeed diffuse through drywall due to it being highly porous. However, parts of the research indicated that the CO permeating the drywall was based on certain thicknesses of the material.
This research further proves the need for CO monitoring throughout any building involved in an incident. Make sure to maintain scene and rescuer safety even after ventilation of the building has been initiated.
Sometimes a question will arise on scene regarding when the building is deemed safe to release back to the occupants. The safest answer to this is when the monitors have a CO reading of zero. Aside from getting the CO level to zero with mitigation procedures, you should also be making sure the O2 levels are between 20-21 percent. If your monitor is reading zero for the CO level and normal O2 levels, the building can be deemed safe.
If your agency has a specific guideline or regulation regarding CO emergencies, make sure you follow your agency’s operating guidelines. Don’t feel pressured to prematurely release the building back to the occupants when there are still CO levels being picked up on the monitor. Understandably the occupants will be eager to get back inside, but to spend that extra time to make the building safe from CO goes much further when it comes to your agency’s handling of the call. Hopefully this will prevent having to go back to the same building later for a medical call because there were residual CO levels still in the building that were missed. Or going back to the same building later on for an explosion, because CO is flammable.
- Humpday Hangout: Close Encounters with Carbon Monoxide
- Exposing an invisible killer: The dangers of carbon monoxide
- Frank Montagna: Carbon Monoxide Alarms
- CHRONIC CO POISONING IN FIREFIGHTERS
Treatment of CO Patient
As was mentioned earlier in the article, CO is a systemic asphyxiant, because it binds to the hemoglobin more readily than O2. There are a few terms to be familiar with when dealing with chemicals and their effects on the body. The first term is “toxidromes.” Toxidromes (or toxic syndrome) are the signs and symptoms that are present due to poisoning related to a specific class of poisons. The next term is “toxicodynamics.” This is what the poison’s cellular and molecular mechanisms will do to the body. The other term is “toxicokinetics.” Which refers to what the body does to the poison and whether it was absorbed, distributed, and/or eliminated from the body.
The chart below emphasizes the importance of first responders wearing proper PPE, especially proper respiratory protection before making entry to remove a victim from a toxic environment to begin proper treatment. Some numbers to keep in mind for rescuers to determine if a victim has had a significant exposure is if a non-smoker has levels >5 percent and a smoker has >10 percent, then those readings are considered a CO poisoning. Regardless, symptoms from a CO exposure can be present with readings on a pulse CO-oximeter between 10-20 percent. Remember normal, CO levels can range from 0-3 percent with no indications of an exposure.
| Parts Per Million of CO | Exposure Times Until Death |
| 400 ppm | Life-threatening in three hours and beyond |
| 800 ppm | Within 2-3 hours |
| 1,600 ppm | 1 hour |
| 6,400 ppm | 30 minutes |
| 12,800 ppm | 1-3 minutes |
Follow local and state protocols when treating hazmat patients. The information below is only a guide, and is not to take the place of standing medical protocols for your department.
Once the patient has been removed from the hot zone, perform the following:
- Assess airway, breathing, circulation.
- Rescuers in proper PPE should provide a patient assessment and remove the patient’s clothing, if deemed necessary.
- Check for respiratory effort and rate and assist with ventilations as needed. 100-percent oxygen is highly recommended. Note: Half-life of CO at room O2 levels of 21 percent is six hours, but if 100-percent O2 is delivered to the patient (via non-rebreather mask or bag-valve mask) then the CO half-life is 90 minutes.
- Check for pulse, and if present, place the patient on the ECG monitor and evaluate the rhythm.
- Attempt venous access.
- If patient has been exposed to CO, then attaching a regular pulse oximeter can give false readings of the patient’s true SpO2 levels. A pulse CO-oximeter device with a finger probe that is designed to strictly look for CO poisoning in the patient’s blood is highly recommended. Note: Make sure to keep the finger probe cover on the pulse CO-oximeter, because the cover keeps ambient light out of the finger probe to allow a more accurate CO level reading.
- Attempt to do as much supportive care as possible for the patient en route to the appropriate hospital. Note: Hyperbaric O2 treatment may be necessary.
Something else to keep in mind if responding to an emergency call where someone is attempting suicide by ingesting a chemical or an accidental chemical ingestion (mainly children). The chemical to keep an eye out for on this type of call is methylene chloride. This chemical can be found in paints, adhesives and paint strippers. These are just a few examples of very common sources for methylene chloride to be contained in, but it can be present in a number of other products. If this chemical is ingested the liver will try to metabolize it with the end result being the metabolite of CO. Rather than inhaling CO, which is a poisoning from the outside-in, this is a CO poisoning from the within the body. Not only is proper medical treatment needed for these patients, but proper decontamination is required, especially if the patient begins vomiting after ingesting the chemical. The vomit will off gas the chemical and can cause secondary exposure on the outside of the patient’s body and to first responders.
CO Response Review
- Secure and size up any scene that involves an active audible CO alarm, or potential/visibly unresponsive people or animals inside of a vehicle, residential, or commercial building.
- Request a hazmat team response.
- Make sure all crew members in the warm zone and any making entry into the hot zone are wearing proper PPE in the form of bunker gear (due to flammability), and SCBA (due to toxic inhalation). Also, make sure that all crew members making entry have had a safety check of their PPE and equipment. Note: Consider potential explosion hazard.
- Request wind speed and direction from the 911 dispatcher. This will aid with rescuers maintaining a safe distance from the scene. This will also aid in any decisions for an evacuation of civilians in the vicinity of the incident.
- A fresh air calibration on the 4- or 5-multigas monitor must be conducted away from the incident and performed by qualified personnel. Also, the air needs to be evaluated for the potential of fire or explosion. Unless rescuers have intrinsically safe radios, then radios will have to be turned off in or around the hot and warm zones.
- If visible, viable victim are located inside a vehicle or building, then the rescuers can perform a line-of-sight rescue. If the victims are deemed deceased, then the incident is considered a crime scene and first responders should try to maintain the integrity of the scene for investigators.
- If the victims are still alive, rescuers should keep in mind that they are hazmat victims and should be removed immediately from the toxic environment. Decontamination of the patients will be required (if deemed necessary) prior to transport to the hospital.
- If there are no victims affected, but CO is present at the scene, then hazmat technicians can perform atmospheric monitoring and mitigation procedures (i.e. ventilation of a building).
Once the hazard on scene has been mitigated, then rescuers must make sure an area is established to conduct thorough decontamination. Not only is decontamination important, but any first responders that made entry into the warm and hot zones needs to be medically evaluated. This can be performed on scene using a pulse CO-oximeter to make sure there is no CO poisoning of the first responders.
CO is a very common emergency call that affects every area of the country. Whether it is accidental or intentional, spring or winter, we will be called to the scene. Sometimes firefighter complacency on these particular calls can get us hurt. As first responders, we must always maintain a sense of vigilance for our safety first so as to effectively be able to rescue and medically treat someone exposed to CO. It is imperative to maintain scene safety and try to get as much information from the 911 dispatcher (while en route) or from on scene observations. The public expects us to be professional and figure out how to deal with the problem, whether it is something small or a major incident.
Robert Bohrer is 19-year veteran of the fire service in South Florida and serves with Fort Lauderdale (FL) Fire Rescue on their hazmat team. He previously served with Palm Beach Gardens Fire Rescue and held the rank of driver engineer. He holds an associate’s degree in emergency medical services from Palm Beach State College. He is a state of Florida-certified Hazmat Technician, Fire Officer II, Fire Instructor II, Live Fire Training Instructor, and holds all TRT Technician level certifications. He also travels all over the state and country offering hazmat and fire training.

