
The call started out as another annoying natural gas call where nothing bad ever happens—until it does. The contractor struck a gas main under the road with a boring device and gas was blowing out of the excavation near the curb. We evacuated nine homes and had been on the scene 43 minutes. The utility techs reported their meters showed gas leaking out of this locked house and asked my captain to force the door so they could go inside. Captain Kenny Patterson radioed to command for a team with the irons (I had the four-gas meter and a six-foot hook). We were near the front door when the house exploded.
In slow motion, I saw it come apart in front of me. The white thing that flew over my head was the front door; it landed across the street. I remember seeing my boots about four feet off the ground. The next thing I saw was the curb at the edge of the street about 30 feet from where I started. Blood was dripping from my face onto the street, my helmet was long gone, and I was burned and trying to figure out if I was alive.
My captain was on the ground about 60 feet from me. I was sure he was dead. He was unconscious when he was pulled from under the rubble. He suffered multiple broken ribs, burns, a shoulder injury, and broken vertebrae. He was out of work for 13 months.
RELATED FIREFIGHTER TRAINING
Tactical Procedures for Responding to Natural Gas Emergencies
Improving Response Procedures to Natural Gas Emergencies
Natural Gas Emergency Strategy and Tactics
Training for Natural Gas Emergencies
The saddest and most frustrating aspect of this event is that this is not a unique case. Unfortunately, there are many similar examples of gas-filled buildings triggering an explosion.
On July 20, 2018, in Sun Prairie, Wisconsin, a contractor hit a gas main, causing an explosion that killed Firefighter Cory Barr.1 On September 27, 2016, in New York City, a gas explosion killed Fire Department of New York Battalion Chief Michael Fahy.2
Firefighters respond to many gas emergencies and usually walk away safely. However, complacent behavior is a normalization of deviance that one day may cause a fatality.

(1) At natural gas emergencies, proper size-up with the right instruments can keep firefighters out of the kill box. (Photo by Dominick D’Alisera; all other photos by author.)
I survived this explosion; the outcome of such gas explosions, however, often is not good. Our medical costs exceeded $1 million. Legal fees were 10 times higher.
What Went Wrong?
After my release from the trauma/burn unit, I asked my friend and Hazmat Coordinator Dan Moran, “What went wrong? I thought I knew what I was doing! This sure wasn’t the outcome that I planned for.”
After many hours of conversation, we determined the following: I did not know what I did not know. This article will help fill some of that same information void for you. Like many other firefighters, my training was incomplete in the area of natural gas emergencies. The goal of this article is to help you improve your skills in sizing up natural gas emergencies by increasing your understanding of the following:
- Underground migration of natural gas.
- Your combustible gas indicator.
- The value of using an explosive gas meter.
- Some practical tactical tools/solutions you can use in your home department.
A key aspect of size-up is knowing the basic properties and tendencies of natural gas. I was repeatedly taught that venting gas is a good sign. Natural gas is lighter than air; therefore, it rises and dissipates quickly. Although that is generally true, in the scenario above, the ground’s frost layer and the asphalt in the street kept the gas from escaping through the soil. Therefore, the gas took the path of least resistance—it migrated underground until it found a route into the house. The force of the explosion proved it was right in the middle of the flammable range (about 10% gas in air), the most forceful explosive mixture possible. During the 43 minutes from dispatch until the explosion, a lot of gas accumulated in that house.
The Properties of Natural Gas
By Frank Montagna
The properties of natural gas, described below, are not news to us. We learned about them and have been reminded about them over and over, but do we appreciate the significance of each as we respond to and operate at natural gas incidents?
Colorless
Natural gas is colorless. We can’t see it. It can fill a room or be leaking up out of a hole in the ground, and we can be unaware of its presence and danger.
If high-pressure natural gas is leaking from an underground pipeline, we may see dirt and dust being blown out by the escaping gas. If you see this, you should realize that gas is contained in the dust cloud and may extend even farther than the cloud and that the cloud can ignite if it encounters an ignition source.
Odorless
Although it is true that the gas has an easily recognizable odor, it is not inherent in the gas itself. The odor is caused by an odorant, mercaptan, also known as methanethiol, which is added by the utility. The odor is pungent and is described as a sulphur or a rotten egg odor. It is usually added to the gas distribution pipeline when the gas is delivered to the utility. This means that it is absent in the transmission pipelines running from where it comes out of the earth to where it is received by the utility. A leak in these pipelines will not be identifiable by odor.
The reason mercaptan is added to natural gas in the United States and Canada is the New London (TX) School Explosion that occurred in 1937. At this disastrous incident, 295 schoolchildren and teachers lost their lives when natural gas delivered to the school leaked and collected in the crawl space beneath it. Because it was not odorized, it went unnoticed until it ignited, destroying the school and causing the deaths. In the aftermath, a gas odorization policy was adopted nationally.
Unfortunately, the odorization of natural gas is not a permanent solution. It is possible for the odorant to be scrubbed out of the gas leaking underground. As gas migrates underground, the dirt acts like a filter removing all or some of the odor. The result can be odorless gas entering a building. Building occupants and responding firefighters might not have the traditional odor of gas warning of a hazardous situation.
Although the natural gas that is now delivered to our home has an easily recognizable odor that serves as a warning of its presence, not everyone can smell the odor. Most people will self-evacuate when they detect a strong odor of gas. Unfortunately, not all can detect it. It is reported that approximately 2% of the people in North America have a sense of smell disorder. These disorders increase with age and, if severe enough, they can prevent those with these disorders from detecting the presence of natural gas, which leaves them in a dangerous position. Additionally, the incapacitated, the very young, and the elderly may not be able to evacuate, and gas is not detectable when you are sleeping. Also, exposure to natural gas can desensitize one to its odor and, after a time, an exposed person may no longer notice the odor. In large buildings, gas may be leaking in only one portion of the building and the occupants remote from this area may not be able to detect its presence and so will not self-evacuate. Should the gas ignite or explode, these people can still be at risk from a fire or an explosion even though they are remote from the leak.
Tasteless
Natural gas has no taste, which makes it more difficult to detect without a combustible gas indicator.
Nontoxic
Manufactured gas use preceded the use of natural gas. It was a mixture of hydrogen, methane, and carbon monoxide (CO). We know that CO is highly poisonous; so was manufactured gas, but it burned well and was used for heat, cooking, and lighting up until the 1940s, when it began to be replaced by nontoxic natural gas. A manufactured gas leak, without ignition or explosion, could result in the death of sleeping building occupants, and opening the oven door, sticking your head into the oven, and breathing the escaping gas could cause death by suicide. We do not have those problems with natural gas. If, however, enough gas fills a room, it, like any other gas, can replace the oxygen we need to survive and cause death by asphyxiation. CO is still a problem with natural gas because incomplete combustion produces natural gas. A poorly vented or unmaintained gas appliance can pump deadly CO into a room or building, putting occupants at risk.
Lighter Than Air
Another well-known fact is that natural gas is lighter than air. If it escapes from a pipe, a furnace, a stovetop burner, or a pilot light and is not ignited, it will rise. If confined and leaking, it will collect and may reach its explosive limit or above. If it is below the explosive range, all that is missing for an explosion is more gas and an ignition source. If it is above the explosive range, all that is needed for ignition or explosion is for enough of the gas to vent out of the building, bringing it back down into the explosive range and the presence of an ignition source. It may be in the explosive range in one part of a building but not in another.
Flammable and Explosive
Natural gas can ignite and burn as well as explode. For it to ignite, it must be present in specific quantities. The right amount is between 5% and 15%. Below 5%, it is too lean to burn or explode. Above 15%, it is too rich to burn or explode. If the gas is in the flammable range, it can ignite, but for an explosion to occur, it must be confined. By confined, I mean in an enclosure. The enclosure can be a room, a house, a shed, a car, a manhole, a silo, or any other type of enclosure.
When the gas ignites, it burns rapidly and heats up the air. The heated air expands, and the rapid expansion of the air is severe enough to knock down walls and blow the roof off of buildings. The force and damage of the explosion will depend on the amount of gas present and the confining structure; 10% gas will produce the maximum explosion.
In buildings housing explosive processes, explosion venting is used to lessen the effect of a possible explosion. Easily blown out walls, windows, or roofs allow the force of the explosion to exit the building, leaving the rest of the structure unharmed. In the absence of explosion venting, a gas explosion can have the devastating results we see on the evening news, often resulting in the total destruction of a building.
When gas in a building is too rich to burn and firefighters ventilate the building, the gas will flow out of the vented openings and the gas level will be reduced. As the level of gas present continues to fall, it will pass down into and through the explosive range before it vents to a safe level. If, as the gas comes down to within the explosive limits, it encounters an ignition source, it can ignite and explode (Figure 1).
Figure 1. Explosive Range of Natural Gas
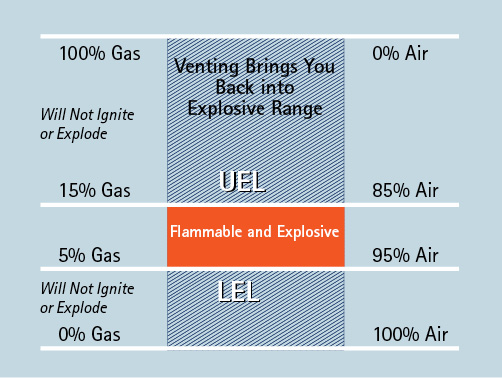
Source: Author.
You should know that the gas meter typically used by the gas utility reads in the “percent of gas in air scale.” This means that its meter reads the exact amount of gas present in air. A 5% reading on the utility meter equals 5% gas in air. The fire department meter reads in the “percent of the lower explosive level (LEL) scale.” When the meter reads 20%, it means that there is 1% gas in air present. At 100 percent, there is 5% gas present in air. Additionally, the fire department gas meter maxes out when it reaches the LEL of 5%, and if the gas is more than the lower explosive level, it will give a display indicating it is off scale and will not display any higher values (Figure 2).
Figure 2. Utility vs. Fire Department Gas Meter Display
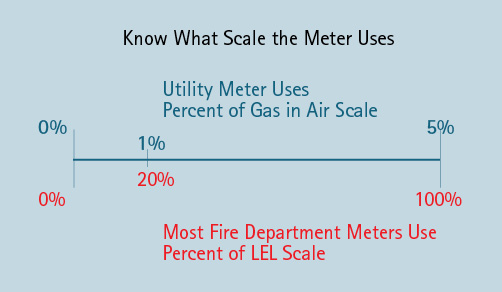
Source: Author
Ignition Source
Outdoors, there are a number of ignition sources that can ignite natural gas: internal combustion engines of passing vehicles and power tools; the flashing lights on fire trucks; sparks created by opening and closing electrical switches on tools and streetlights, matches, or any other flame; and the list goes on.
Indoors, there are plenty of ignition sources. When we ring the doorbell, we introduce an ignition source. When we cut a lock with a saw or create a spark with a tool, we provide an ignition source. Opening the entrance door can admit cold air, triggering a furnace or boiler to fire, providing an ignition source. Operating any electrical switch creates a spark unless it is intrinsically safe in an explosive atmosphere. An open flame and static electricity from walking on a rug are ignition sources. You get the idea; there are a lot of ignition sources both inside and outside.
Migration
If gas is escaping outside, unconfined, it will rise into the atmosphere. As long as it does not encounter an ignition source, it will not ignite. Natural gas, being lighter than air, wants to rise, but there are things that can affect its ability to rise. A thick canopy of branches and leaves over the leak can trap the gas and slow its rising, and a strong horizontal wind can blow the gas some distance parallel to the ground before it is able to rise high enough not to be hazardous. Gas leaking underground will rise through the ground into the air unless it is prevented by pavement, ice, or snow. It will leak up through puddled water and can be evidenced by the gas bubbles popping on the water’s surface. Even a frost on the pavement can prevent the gas from escaping. Gas trapped underground will travel underground some distance and can find its way into surrounding structures and underground enclosures like electric vaults and sewer systems.
Just like responding to a structural fire, where knowledge of fire spread and building construction is vital knowledge needed for your decision-making process on what to do and what not to do, the properties of natural gas are vital information when responding to gas leaks. Everyone responding, from the incident commander to the new probationary firefighter, needs this information. See that they all get it.
FRANK MONTAGNA, a 43-year veteran of the Fire Department of New York, retired as a battalion chief, a position he held for 26 years. He was assigned to the department’s Training Academy, where he was responsible for curriculum and officer development and simulation training. He, in collaboration with Con Edison’s Emergency Response Group, wrote the department’s gas and electric procedure manuals. He is one of the creators of Fire Engineering’s simulations. He has a BS degree in fire science. He taught a course for John Jay College based on his book Responding to Routine Emergencies, has published numerous fire-related articles, and frequently lectures on these topics.
Another Unknown
Natural gas is combustible between 5% and 15% gas in air. Most four-gas meters use the lower explosive limit (LEL) scale to measure the combustibility of the sample passing through the meter. In the LEL scale, 100% LEL on the instrument’s display tells the user that the concentration has reached the LEL, which is equal to 5% gas in air. Readings on the meter start at 1% LEL and top out at 100% LEL. You rely on the LEL readings to warn you as the concentrations approach combustible levels.
The four-gas meters firefighters typically carry can have a couple of critical blind spots. Many of them can detect only on the LEL scale. That means that they are blind to readings below 1% LEL and they do not display how far above 100% LEL the gas concentration has reached. At the low end, gas may be present, but the meter is not sensitive enough to detect it. On the high end, the meter does not tell the user how far above 100% LEL the gas sample may be. These factors create a potential hazard for the firefighter on scene. For these reasons, look at the widely used four-gas meter as a tool that gives us only a partial size-up picture.
Sensors and Detectors Used for Size-Up
Five main gas sensor technologies are used in gas detectors commonly used by fire departments: wheatstone bridge, metal oxide sensor (MOS), catalytic bead, electrochemical, and laser.
Wheatstone Bridge
This type of sensor has been in use for decades and is used in many combustible gas indicators (CGIs). The gas sample is pumped into the unit and across a heated coil that causes the gas to burn. The increased heat from the burning sample causes an increase in electric resistance. The resistance is compared to a reference coil out of the gas stream. The difference in electrical resistance is converted into an LEL reading on the meter’s display.
MOS
Detectors with MOS, known as explosive gas detectors, are growing in popularity in the fire service, but they are still underused. Their strength is that they can detect very low levels of combustible gases almost instantly. Inside the sensor, the flammable gas sample reacts with a thin piece of metal oxide in a chemical oxidation or reduction reaction. Using the resulting difference in electrical resistance, the instrument displays the measured amount of gas on the digital display or with audible tick sounds. Faster ticking means more gas is present.
The advantage of the MOS in explosive gas detectors is its ability to instantly detect gas at much lower levels than the catalytic bead or wheatstone bridge type of sensor used in many four-gas meters. Thinking back to the incident at which I was injured, if I were carrying an explosive gas detector with an MOS, I would have gotten an instantaneous warning of gas seeping from inside the home. It likely would have changed the incident to just a close call instead of a life-threatening event.
The limitation of the MOS is that it can detect as low as about 10 parts per million (ppm). For reference, 1,000 ppm is equal to 0.1% gas or 2% LEL. On the job, this means that the explosive gas meter (MOS) will react at 10 ppm compared to the first possible reading on your four-gas meter at 1% LEL or 500 ppm. If you carry only a four-gas meter, you will be blind to any changes in concentration from 10 ppm to 500 ppm (1% LEL). Keep in mind that most humans can smell the odorant in natural gas at 1 ppm or less.
Explosive gas detectors typically pair their MOS to a ticker that alerts the user to small changes in gas concentrations. As concentrations increase, the tick speeds up. This is especially useful when trying to find the exact source of a leak. Some units also have digital displays that automatically switch from ppm to percent LEL. Their ability to respond to small concentrations provides valuable information to firefighters that is not available with the standard four-gas meter.

(2) This explosive gas detector uses a metal oxide sensor, reads very low levels of many explosive gases, and automatically switches from ppm to percent LEL.
Many natural gas utilities use instruments that include an MOS in addition to the usual four-gas configuration. Generally, explosive gas detectors are affordable, costing much less than a typical four-gas meter. Pairing the two is a great solution for firefighters. MOS units are inexpensive and reliable.
Explosive gas meters (MOS) also respond instantly to spilled or leaking flammables like gasoline and propane. This makes them very useful for firefighters. The sensor also responds to acetone, alcohol, ammonia, butane, ethylene oxide, gasoline, halon, hydrogen, industrial solvents, jet fuel, lacquer thinner, methane, and propane. From a practical perspective, the explosive gas detector will alert the user that there is a combustible gas present, but it doesn’t specify what kind.
Catalytic Bead Sensor
The catalytic bead sensor has mostly replaced the wheatstone bridge sensor in the detector market and has some similar operating principles. Noted author of several books on hazardous materials Chris Hawley describes this sensor as follows: “Instead of twists of wire forming a bridge, the catalytic bead sensor uses a bowl-shaped string of metal with a bead of metal in the middle. The metal is typically coated with a catalytic material that helps burn the gas sample off efficiently.”3 The burning or oxidation releases heat, changing the electrical resistance of one element over the other, which is translated into a numerical reading on the display.
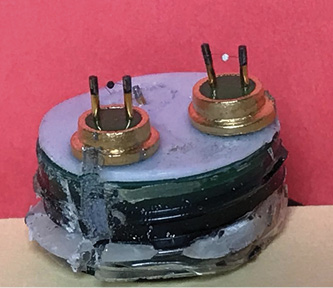
(3) Catalytic bead sensors are accurate and read from 0% to 100% LEL. Many manufacturers produce instruments with these sensors that read both ppm and LEL on the digital display.
Detectors using catalytic bead sensors employ different methods to protect them from damage in high concentrations of gas. It is tactically important to note that the detector shuts off to protect itself. Detector brands on the market display this function in different ways. Some display “OL” (over limit), “999,” “***,” or “OFF” (off scale) when concentrations exceed 100% LEL. One brand displays a skull and crossbones when readings get above 10% LEL. Knowing how the detector responds to different concentrations is critical to making the right size-up decision at gas emergencies.
Catalytic bead sensors can be compromised. The catalyst is a chemical that speeds up the burning of the gas sample and can be eroded, consumed, or poisoned, resulting in false negative readings. Some examples of potential poisons are lubricants, silicone, and some adhesives. These compounds can change the sensitivity of these sensors. For firefighters, this means keeping the gas detector away from cleaning products (including the leather and vinyl cleaners often used in the rigs), seals for gaskets, polishes, cosmetic or medicinal hand creams, caulking, and tetraethyl lead in gasoline and most lubricants. It also means not to “test” or train with this instrument using the exhaust pipe of your chief’s car.4
Electrochemical Sensors
Most four-gas meters also include electrochemical sensors to detect carbon monoxide (CO), oxygen, and hydrogen sulfide. It is important to understand the interaction of electrochemical sensors with combustible gas sensors.
The chemistry and design of electrochemical sensors are different from those used to detect combustible gases. Explained simply, the sample is exposed to the sensor head passively or by a pump. The sensor head contains chemicals that react with the target gas. This reaction generates an electrical current and is shown as a digital reading in ppm on the display
Review your instrument’s operation manual for its T90 time—the time it takes for a sensor to reach 90% of its final, stable reading. The lesson here is that sensors respond at different speeds and can vary for each gas target, including combustibles.
Consult the manual also to learn the cross-sensitivity of the sensors. For example, CO sensors are cross-sensitive to hydrogen. I remember a call where we responded to the report of a CO alarm in a small warehouse. Our meters showed CO was present, but there was no obvious source. Knowing this cross-sensitivity allowed me to recognize that the CO sensors were reacting to hydrogen being driven off by the battery chargers in the warehouse forklifts.
CO sensors are typically cross-sensitive to acetylene as well. So, if you get a CO reading at an industrial occupancy or where you think there may be leaking acetylene, considering this cross-sensitivity is important.5
The oxygen sensor is a key component of the four-gas meter. Obviously, it monitors the breathing air in the environment in which you work. The normal reading for oxygen is 20.9%. If that reading decreases, the user knows that something else—a combustible gas, CO, or some other toxic gas—is replacing oxygen in the room. However, the oxygen sensor is also important because reduced oxygen levels may negatively affect the performance of the sensors for combustible gases. For example, the LEL sensor in many detectors needs an adequate supply of oxygen to provide accurate readings. Less oxygen results in less efficient burning, which yields false low readings for combustible gas. When using a four-gas meter, actively monitor all sensor readings to determine the situation.
Laser Gas Sensors
Natural gas utilities have been using tuned lasers to detect gases for the past 15 years for their mandated leak surveys. Recent improvements in the technology have made it possible to make these detectors smaller and more compact. The technology has great promise for the fire service. These handheld detectors typically have a range of about 100 feet and can detect methane gas in the 5- to 50,000-ppm range. The laser is tuned to natural gas and, when it passes through the plume, some laser energy is absorbed by the gas. The instrument analyzes the difference in energy sent out and reflected and provides a digital readout. It can make your size-up faster, more effective, and safer.6
Size-Up Training
Classroom training is fine, but for firefighters to really understand how the various detectors work, they must train with them under actual conditions. Watching how the various meters react and alarm, interpreting their readings, and then witnessing the gas ignition are valuable training and best done using a tabletop explosion chamber.
Model House Exercise
At the Rockland County (NY) Fire Training Center, a small model house used as a training aid enables firefighters to use their own meters in small-scale, realistic meter exercises during which they can see and interpret readings (photo 4). First, a small amount of natural gas is introduced into the house. The trainee puts the meter into the building and observes the reading below the LEL. Trainers then ask questions such as, “Will it ignite?” (photo 5). The ignition source is activated, but the gas does not ignite because it is below its LEL.
Next, a calibrated amount of gas is added to the house to create an explosive atmosphere. The trainee puts his meter back into the house and now sees a reading in the flammable range (photo 6). We proceed to discuss the reaction of the meter and its alarms. The trainers pose more questions about combustibility levels and standard operating procedures (SOPs).
Finally, the ignition source is activated, and the gas ignites. This creates pressure in the house, blows open the door, and lifts off the roof (photo 7). In the real world, walls typically move outward, creating a void at the corners of the building, much like collapsing walls do. The discussion with the trainees continues and focuses on the effect of pressure during an explosion and how the pressure causes real buildings to explode and collapse, creating a kill box for firefighters.
Several tactically important points relative to size-up are reinforced in these demonstrations. Trainees are taught the following:
- What their meter displays and alarms show as the amount of gas increases.
- How the readings relate to the action level dictated in their SOP and the steps firefighters are expected to take at these levels.
- Building evacuation decisions.
- Other actions they should take in accordance with their SOPs.
Sensors Aid Size-Up
Understanding the critical details of your meter is vitally important to your safety and success on the scene. Add this information to your size-up. Following are a few suggestions that will help to improve tactical size-up of natural gas emergencies. These scenarios have many variables, and you should always rely on your SOPs, which must be based on best practices of the natural gas industry, a solid understanding of your tools (combustible gas instrument), and the behavior of natural gas during emergencies.

(4) Explosion chambers allow firefighters to use their own meters in real natural gas environments.

(5) A trainee uses a CGI to measure gas at levels below the LEL inside the model house.

(6) The CGI reads in the flammable range and shows a respective drop in oxygen content. Note: This device automatically transitions from the LEL scale to the % gas scale.

(7) After additional natural gas is added to the house, the ignition source is activated and a dramatic deflagration of gas causes an increase in pressure, blowing open the door, jetting fire out, and lifting the roof of the house.
The Locked-Building Scenario
Your mission during natural gas emergencies is life safety—not find and fix. As we know, natural gas can migrate underground and seep into buildings through various points of entry for utilities (water, sewer, gas, electric, steam, communication lines), through cracks in the soil or foundation, and through noncompliance with the plumbing code (pipes without or dried-out traps).
Locked buildings are an excellent application for an inexpensive explosive meter that uses an MOS. As mentioned earlier, these detectors react instantly to very low levels of gas. I call locked buildings with explosive gas and numerous ignition sources improvised explosive buildings (IEBs).
Most utilities have recognized the extreme hazard of locked-building situations and have procedures to shut down electric and gas and wait for the building to vent down on its own while firefighters keep utility and fire crews out of the kill box. This may take six to eight hours for a suburban home. Since firefighters want to fix everything quickly, we often force the door so we can help the utility vent the building of explosive gas, find the source, and go home. Often, we rationalize the extreme risk we take by forcing the door this way, “We need to get inside and search”—as if it were a fire. Ask yourself this, “Have you ever in your career heard of someone getting asphyxiated by natural gas?”
The real question is, is anyone inside? The odorant in natural gas is very strong and can be detected by most people at very low levels—well below the LEL—and their reaction is to leave the building. Additionally, experience substantiated by the Centers for Disease Control and Prevention has shown that humans get nauseous and headaches from even low levels (4 ppm for several hours) of natural gas and the odorant.7 So, the questions are, what are the odds that occupants are still inside, how extreme is the risk to firefighters, and is there an alternate method we can use to resolve the life safety concern?
Tactical Agility
There is a balance between the application of the classic “risk a lot to save a lot” condition in this situation and in a fire situation. We must strategically and tactically understand that this is not a search in a fire condition. Searching firefighters cannot read the explosive gas concentrations except for the area in which they are; we cannot alter conditions in a potentially explosive situation as we can in a fire. We have absolutely no control over gas concentrations and ignition sources and surely cannot control the explosion with a hoseline. It goes “boom” in a microsecond, often with fatal consequences.
A sensible alternative to a “rapid risk-a-lot primary type search” is to first try to determine if anyone is home. Obviously, this is tactically different from what we do for fire conditions. Although the information will not be 100% conclusive, ask neighbors about the locked building occupants: Are they away on vacation? Do they work? Can you call them at their job? Are police watching their house when they are away? Can you alert them with the loud speakers, sirens, or air horns on your trucks? In an urban environment, the risk/benefit equation may be different. Forcing doors on apartments in multiple dwellings may be necessary and worth the risk.
The laser-based detector is another valuable tool for locked buildings. Although field experience is currently accumulating, it appears that laser detectors can and will reveal natural gas through glass in windows and doors and leaking from around doors and windows.
If your explosive gas detector indicates that gas inside is seeping to the outside, remember that life safety is your primary mission. There likely is no safe way to enter this building. All options put firefighters in the kill box if the explosive gas inside finds an ignition source. We know the building contains explosive gas. We know it contains ignition sources. We just don’t know when the gas concentration will enter the flammable range.
Unbelievably, some utilities still ask firefighters to take the extreme chance and force the door to allow utility crews inside. Keep in mind that your turnout gear is not rated for explosions and that you have no plan B if the building lights up (explodes). It is also important to remember that the fire department, not the utility company, is responsible for the life safety of everyone on the scene.
Outside Odor of Gas
Sizing up outside odors of natural gas can be challenging. Dispatch information often reports an odor near an address. Experience tells us that the source could be from an underground main or a service line leak, escaping gas from an interior leak in a nearby building, exterior gas piping, or meters nearby. It could be from a pressure-relief device on a distribution system or a transmission line. The firefighters on site must size up the situation, determine if there is a life safety threat, and develop the best strategy and tactics. Using their instruments, firefighters on scene must determine the following: Where is there a lot of gas, where is there a little, and where is there none?
This is a prime application for the laser methane detector. It can quickly help determine if gas is rising out of subsurface structures like sanitary, storm, or electric or steam manholes. The laser can also help remotely survey nearby buildings and other possible leak sources. This new technology will also help your size-up by eliminating wasted time by determining where gas is not present. Once possible leak sources are identified, use the CGI to determine concentrations to provide further situational awareness and develop tactical objectives.
If the laser detector is not available, the next best thing is an explosive gas detector with an MOS, which, as noted above, will provide instant indications of very low levels of gas. This will expedite size-up and help determine the extent (if any) of the migration of gas.
Inside Odor Calls
The four-gas meter is typically used for inside odor calls. Recall that the catalytic bead sensor in these devices will not detect levels below 1% LEL (500 ppm). This presents a significant blind spot and risk to the responder. The meter used by most gas utilities can detect (with its MOS) concentrations far below 500 ppm. The MOS-equipped detector provides a response that can be as much as 10 times faster than other instruments typically used in the fire service. It also provides the earliest possible warning of the presence of flammable gases, thereby increasing our safety.
Some departments have a “find and fix” policy. In these cases, the MOS-equipped air monitor is helpful in finding small leaks on appliances so the nearest gas valve can be closed, resolving the problem and maintaining service to the rest of the apartment or building.
This article has highlighted a few critical tactical considerations for your next response to a natural gas emergency. To provide the best possible service to the citizens and to protect the lives of your crew, strongly consider improving your training and understanding of your explosive gas detectors and the behavior of natural gas when released from its piping.8 Finally, your response procedures must be in line and coordinated with the best practices of the utility industry.
References
1. MILWAUKEE JOURNAL SENTINEL, 11 July 2018. Accessed on line. Also. https://emberly.fireengineering.com/2018/07/16/215575/sun-prairie-wi-fire-department-lodd-funeral/.
2. https://emberly.fireengineering.com/2016/09/28/208270/fdny-lodd/.
3. Hawley, Chris. Hazardous Materials Air Monitoring and Detection Devices. Delmar Publishing, 2002.
4. Technical Note 144: “Handling LEL Sensor Poisons,” RAE Systems by Honeywell Subject: Handling LEL Sensor Poisons Keywords: How LEL Sensors Work, LEL Sensor Poisoning, LEL, sensor, poisons, operation, precaution, handling, assembly, service. Created Date: 6/10/2014 4:01:29 PM; https://www.raesystems.com/sites/default/files/content/resources/Technical-Note-144_Handling-LEL-Sensor-Poisons_03-99.pdf.
5. Rae Tech Note 114. “Sensor Specifications and Cross-Sensitivities,” raesystems.com, jmtest.com/PDFs/RAE-Systems-TN-114-Sensor ….
6. Knapp, Jerry, “Remote Gas Detection with Handheld Lasers,” Fire Engineering, Nov 2019, 65.
7. Clayton and Clayton 1981, www.atsdr.cdc.gov, Tox profiles/tp172-cfpdf.
8. Refer to Knapp, Jerry and Daniel Moran, “Improving Response Procedures to Natural Gas Emergencies,” “Natural Gas Emergency Strategy and Tactics,” and “Training for Natural Gas Emergencies,” Fire Engineering, March, April, and May, 2017, respectively.
JERRY KNAPP is a 44-year firefighter/EMT with the West Haverstraw (NY) Fire Department and the author of House Fires (Fire Engineering). He is a training officer with the Rockland County Fire Training Center and chief of the hazmat team and a technical panel member for the Underwriters Laboratories research on fire attack at residential fires. He is the author of the Fire Attack chapter in Fire Engineering’s Handbook for Firefighter I and II, and has written numerous articles for Fire Engineering.

