By BARB BROWN
Scenario: You are dispatched for a 55-year-old male who was seen lying unconscious through a kitchen-floor window by a neighbor. On arrival, you gain entry and immediately notice several cables connected to what appear to be two batteries and a round box; an additional cable is attached to the patient. As you do your primary survey and open his airway, you see a medic alert necklace indicating that he has heart failure, insulin-dependent diabetes, and hypertension.
On examination, the patient is unresponsive, pale, and diaphoretic, with four to six agonal respirations per minute. You cannot palpate a carotid or radial pulse. The neighbor states that the patient lives alone; he doesn’t know much more and cannot provide any additional medical history or events leading up to the emergency. So, what is your treatment plan? Cardiopulmonary resuscitation (CPR)? Advanced cardiovascular life support? Disconnect the cables getting in your way? Investigate the purpose of the cables? The answer might seem obvious, but keep reading before you make your final decision.
HEART FAILURE OVERVIEW
More than 550,000 people a year are diagnosed with heart failure—an average of one per 100 people ages 62 years and older. As more patients are diagnosed or become sicker, the need for heart transplants continues to rise.
 |
| (1) The Thoratec Heartmate XVE (left) and Heartmate II (right). (Photo courtesy of Thoratec.) |
There are 20 million people worldwide diagnosed with heart failure, five million in the United States alone. Of these 20 million patients, one million have class IV heart failure, or end-stage failure. At this juncture, traditional medical therapy is no longer effective; without a heart transplant, a class IV heart failure patient will soon die. Unfortunately, there are only 3,000 donor hearts available each year, creating a critical “demand surpasses supply” situation.
As traditional pharmacological therapy such as diuretics, digoxin, and ace inhibitors becomes less effective for advanced heart failure patients, and increasing numbers of patients become eligible for heart transplants, mechanical support such as the ventricular assist device (VAD) has begun driving the future of heart failure management.
Welcome to today’s 21st-century heart failure patient population and the challenges that this new patient population presents for fire and rescue as they are discharged home and back into the community with cardiac assist devices, the most common being left ventricular assist devices (LVADs). A LVAD is designed to support the once failing heart by surgically attaching a pump to the left ventricle to boost blood flow to the ascending aorta by way of a surgically implanted cannula. A right ventricular assist device (RVAD), designed for right-sided ventricular support, attaches to the right ventricle and flows blood to the pulmonary artery. A driveline exits the patient’s body and attaches to an external power source (portable batteries) and a system controller. The controller operates the internal device and provides the patient with an external alarm panel to make him aware of any issues with the device, display battery status, and alarms for cable disconnections. It can also maintain a daily log of pump activity.
There are two groups of heart failure patients that meet criteria for a VAD: bridge to transplant (BTT) and destination therapy (DT). BTTs are patients with nonreversible heart failure that meet criteria for a heart transplant. This patient population may have its LVAD for a short or long time, depending on the availability of a donor heart and the patient’s priority on the transplant waiting list.
DTs meet class III or IV heart failure per New York Heart Association criteria. They have secondary medical conditions or other contraindications to a successful heart transplant. Contraindications might include obesity, pulmonary hypertension, diabetes, renal dysfunction, noncompliant social habits, and recent cancer diagnosis. To better understand these devices, let’s focus on the generational differences and what has driven the technological changes over the years.
FIRST-GENERATION DEVICES
The first-generation, or “pulsatile,” devices are designed to mimic the way the native heart operates. When the bladder in the pump is full, the pump contracts, thrusting blood flow forward into the aorta. These devices can be driven pneumatically (by air) or by a hand pump in case of pump failure. One common first-generation device is the Heartmate® XVE by Thoratec, one of the most often implanted LVADs worldwide. It holds a low risk of thromboembolic complications and can be used for BTT or DT. The Jarvik 2000 FlowMaker® is another pulsatile device that actually supports cardiac output by working in synchrony with the left ventricle, supporting it as it contracts. A biventricular assist device (BIVAD) assists the unique patient population that requires both right and left ventricular support, and it can have two pumps physically outside or inside the patient’s body. BIVADs are also pneumatically driven by an external power source and considered pulsatile flow devices because they produce palpable pulses. If the device loses battery power, patients or providers have an alternative way to operate the device by using two bulbs connected to the ports where cables normally supply channel power to the device. Once the bulbs are connected, the operator squeezes one bulb at a time rapidly to maintain flow through the device and through the heart. If you are using a hand pump or bulbs for a pneumatically driven system, squeeze and release the hand pump or bulbs at the same rate as you would when doing chest compressions.
SECOND-GENERATION DEVICES
Second-generation devices represent the first leap toward a continuous flow concept. They have an axial flow pump that operates continuously throughout cardiac diastole and systole to propel blood into the ascending aorta. These devices work very differently from the way our native hearts operate. If the device is intended to “support” the patient’s native heart, a weak pulse may be palpable. However, if the patient is completely dependent on the device (i.e., has no ejection from his native heart), you will not be able to palpate a pulse. This may alter your systolic and diastolic blood pressure measurements, Sp02 (pulse oximetry), and EKG rhythms substantially.
How is this possible? The LVAD is now mechanically augmenting the left ventricle and providing the workload once provided by the heart itself. Electrical activity remains intact but is independent of the LVAD, much the same as pulseless electrical activity but with real, bonafide perfusion. If you are capable of obtaining a mean arterial pressure using a Doppler device, this number would be a valuable reference parameter for you.
 |
| (2) An internal view of the Heartmate II, the most widely implanted LVAD today. The rotary pump does not generate pulsatile flow. (Photo courtesy of Thoratec.) |
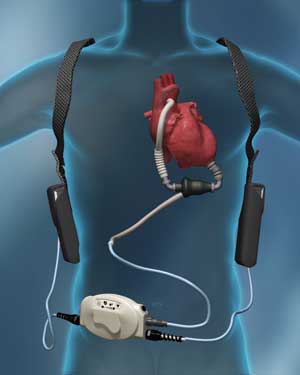 |
| (3) The external equipment accompanying a Heartmate II LVAD. (Photo courtesy of Thoratec.) |
Second-generation devices are smaller, quieter, and more efficient rotary pumps than their predecessors. They can operate as fast as 6,000 to 15,000 revolutions per minute (rpm) while providing seven to 10 liters per minute of circulating blood. The Heartmate II® LVAD by Thoratec has become a leading LVAD, seen in the community today with more than 6,000 implanted worldwide as of October 2010.
THIRD-GENERATION DEVICES
Third-generation devices are also continuous flow and referenced specifically as “axial flow” devices. They are also nonpulsatile, which again implies that patients are likely to have no palpable pulse or measureable systolic or diastolic blood pressure. This, too, is dependent on the percentage of support the patient’s left ventricle needs to provide adequate cardiac output. This generational device’s mechanics are what set it apart from the prior generational devices.
Instead of a spinning rotary pump system that creates an auditory sound similar to that of a turbine engine hum, third-generation VADs operate as a “hybrid system,” with wide impeller blades suspended by passive magnets and hydrodynamic thrust bearings that spin between 2,400 and 3,200 rpm, delivering up to 10 liters of blood volume per minute. Physiologically, this device operates by drawing blood from the left ventricle and propelling it through an outflow graft connected to the patient’s ascending aorta. Because of the design of the suspended wide impeller blades, red blood cells are not damaged and, therefore, have a low risk of clot formation.
FOURTH-GENERATION DEVICES
The fourth-generation device currently in clinical trials takes a completely new approach than previous devices. The total artificial heart (TAH) replaces both native ventricles and valves with a complete artificial heart. Reverting back to pneumatically driven technology design, patients with fourth-generation devices would have palpable pulses and a measurable systolic and diastolic blood pressure. This still requires an external power source and controller panel, but the concept of completely removing diseased ventricles and avoiding native valve complications such as aortic and mitral regurgitation offers a significantly less complicated wait for a heart transplant. A current example of a fourth-generation device would be the temporary TAH by SynCardia Systems, Inc. (photos 4-5).
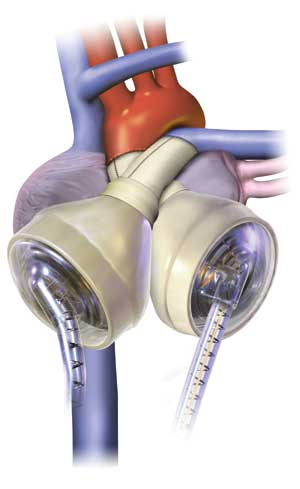 |
| (4) A SynCardia temporary Total Artificial Heart (TAH-t). (Illustration courtesy of SynCardia Systems, Inc.) |
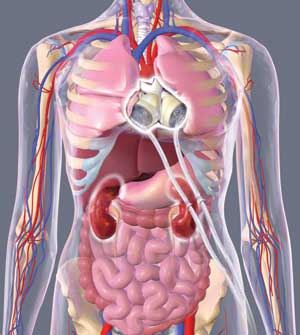 |
| (5) SynCardia TAH-t implanted in a patient. (Illustration courtesy of SynCardia Systems, Inc.) |
Regarding our scenario’s unconscious patient, given the information you now have, do you still suspect a cardiac arrest? With this new information, you probably now recognize that this patient has a LVAD. Since he may not normally have a pulse, what other medical emergencies might cause pallor, diaphoresis, and unresponsiveness with agonal respirations? Examining his medic alert necklace information, you check his glucose; it comes back too low to read. Following your hypoglycemia protocol, the patient shows immediate signs of improvement; he subsequently becomes alert but is slightly confused. Nonetheless, it’s a huge improvement from where you originally started. So, now that you know what a LVAD is and its basic operating principles, how do you assess if the device is working properly?
INTEGRATING LVAD INTO ASSESSMENT AND TREATMENT
What modifications do you need to make when managing this patient population? All protocols and decision making are based on traditional vital signs. The different generational VADs saturating our industry with potentially “pulseless” patients require modified assessments, moving from measured hemodynamic numbers to a “hands-on” patient assessment.
Use alternative ways to assess the adequacy of perfusion such as pale vs. pink, dry vs. diaphoretic, and alert vs. confused. Vital signs have always played a part in our overall decision making. Learn to incorporate the device into your assessment and not let it take over your assessment. An example of patient assessment includes the following.
- A = Airway.
- B = Breathing.
- C = Check for other causes and treat per your local protocol. Listen just below the heart to hear if the device is running, assessing for a palpable pulse.
If there is no palpable pulse detected, consider the following:
- 1. The device is not running. Troubleshoot the device and treat per your local protocol.
- 2. The device is running, but the patient is still unconscious or unstable.
- D = Disability, better known as neurological evaluation. Hemorrhagic strokes are currently the leading cause of death for LVAD patients.
- E = Expose the patient, but be cautious with trauma shears; you don’t want to accidentally cut a driveline or cable exiting the patient’s body that might be hidden under an article of clothing. Assess the dressings over the driveline exit site (found in the abdominal area) for signs of infection. Infections are a huge concern for all VAD patients.
TREATMENT PEARLS
Signs of hypoperfusion. All VADs love volume; they are preload dependent and afterload sensitive. Any efforts to provide fluid volume and reduce afterload will likely be of benefit in restoring adequate perfusion. For basic life support providers, keeping the patient supine and his legs elevated optimizes fluid status. Advanced life support providers can administer intravenous fluids to restore circulating volume. Pressors are generally contraindicated, as they increase afterload, worsening forward flow from the VAD. Assessing for hypoperfusion can be as simple as assessing levels of consciousness, skin color, and temperature or assessing the system controller for messages such as “low flow.”
Trauma. Keep in mind that even VAD patients can suffer traumatic injuries. In cases such as falls or motor vehicle crashes, you may want to incorporate the LVAD and its accessories into your assessment, as these, too, stopped abruptly at what may have been a high rate of speed.
Can you do chest compressions (CPR)? Given the attachment points (heart and great vessels), it’s not recommended, but the final answer lies with your system medical director or from immediate telephone consultation with the VAD coordinator from the implanting center.
Can you use a Kendrick Extrication Device? Again, given the attachment points and pump location, you may dislodge or kink a hose when you tighten the chest straps. Keep this in mind.
Packaging a LVAD patient. Be aware of the cables, controller, and batteries. It may be best to place the spider or stretcher straps under the VAD cables so you are not creating any torque. At minimum, be aware of this extra hardware.
Do these patients take blood thinners? Since this is device specific, patients may or may not be on blood thinners. The safest approach is to assume they are and assess/document a neurological exam.
Can they go to the local emergency department (ED)? If the emergency involves or is the cause for a LVAD, it is ideal that they return to the hospital that placed the LVAD. The implanting facility has the equipment and expertise to manage its VAD patients; it also knows its patients very well. Alternatively, it can provide phone consultation to help with the management of the emergency. If the emergency does not involve the LVAD, I would suggest contacting the LVAD coordinator at the implanting institution and, at minimum, make him aware you are going to the local ED so it can assist if needed.
What equipment should I take with the patient? Ask the patient if he has an emergency bag, backup batteries, or an alternate power source. Make every effort to ensure such items go with the patients in case they are on portable battery power. Depending on the device and age of the batteries, battery life can vary from four to 10 hours before fully charged replacement batteries are needed.
Are there altitude concerns? No.
What resources are available? Every LVAD patient is managed by a VAD coordinator who is accessible by cell phone or pager. The patient and family know this number, and the coordinator is accessible 24/7. Use this coordinator as a resource, but keep in mind that, if the direction provided is outside your protocols, it may be necessary to also consult your medical control.
Protocol development. Develop your own agency or service LVAD protocol to address key questions/decisions BEFORE your next LVAD emergency.
VAD center locations. Know which and how many hospitals in your area are VAD centers and find out which devices they routinely place. Establish a relationship with these VAD centers for guidance in the development of a VAD protocol or policy.
Several years ago, a group of VAD coordinators, EMS managers, and medevac program representatives within the Mid-Atlantic region (Virginia; Maryland; Washington, DC) came together to discuss the future growth of this patient population and how to adequately meet the needs of fire and rescue. This committee, initially called the Mid-Atlantic Regional Pre-Hospital Mechanical Circulatory Support Task Force, developed and has since patented a prehospital LVAD field guide that encompasses the most current devices in the community today. Similar guides have been developed in other areas. To download a copy of the Mid-Atlantic Region publication, visit emberly.fireengineering.com/webxtra.html.
This 2009 field guide is color coded after each device manufacturer agreed to color code its device to the chosen color in the field guide. For example, if you see an orange label on an LVAD system controller, this system is the Heartmate II® by Thoratec. The orange sticker is paired up with the field guide page that has an orange border; it is also written in orange, which are the field guide pages you would use for troubleshooting and emergency procedures. This field guide is designed to address 10 key questions in an emergency situation and provides brief and direct answers for that specific device and is filled with pictures to assist you.
Additional goals of this committee were to provide ongoing outreach of prehospital education in LVAD patient management by way of avenues such as articles, conferences, training, and e-file availability (for media such as the field guide and PowerPoint® presentations). The committee is currently updating the field guide for 2011 to encompass the most recent changes within the LVAD industry and to make it available as an application for various smartphones.
Our scenario patient continues to improve; his level of mentation now resembles a Glasgow Coma Scale of 15. His color has returned; he is now dry and feeling much better. He states that he has only had his LVAD for about four months and is not used to having so much energy, sometimes forgetting to check his sugar or eat. He ensures you that he will eat lunch, feels no need to go to the hospital, and signs your patient transport refusal form.
The LVAD patient population is growing in virtually every community. The patients are often discharged home to a better and healthier quality of life.
This technology and its ever-changing enhancements will continue to challenge fire and rescue personnel, forcing EMS providers to modify traditional assessments and think outside the box in treatment decisions as they learn more about and increasingly encounter this fascinating technology.
REFERENCES
1. www.thoratec.com.
3. Paradigm Series VAD Educational Web site, www.vadparadigm.com/?gclid=C07hnZKPIKgCFQI_5QodVDkmDA.
BARB BROWN, NREMT-P, is deputy chief of EMS for the Dale City Volunteer Fire Department in Prince William County, Virginia, and a critical care flight paramedic for MedSTAR Transport at the Washington Hospital Center in Washington, DC. She has been a clinical educator and conference speaker throughout the Mid-Atlantic region on EMS topics for more than a decade and has played an active role in the development of field resources for fire and rescue personnel for managing patients with left ventricular assist devices.

