TRICHLOROETHYLENE
HAZARDOUS MATERIALS
CHEMICAL DATA NOTEBOOK SERIES #45
TRICHLOROETHYLENE IS a sweet-smelling, clear, colorless, volatile liquid that is classified by DOT as ORMA. It can cause serious injury and possibly death to exposed persons under certain conditions. It is reactive to a large group of chemicals and has somewhat confusing burning characteristics. It is widely used for such purposes as metal degreasing, electronic parts cleaning, textile processing, and dry cleaning, and as an extractant for oils, fats, and waxes. It is also used as a disinfectant, fumigant, heat exchange fluid, solvent, and intermediate in some other products. It is usually shipped in five-gallon and 55-gallon steel drums, in addition to tank trucks and rail tank cars. It is a suspected carcinogen.
PROPERTIES
Trichloroethylene is classified as ORM-A but has a flash point of 90°F. This may be confusing to safety personnel who need to be able to classify hazardous materials according to their physical and chemical properties, so exercise care in the face of conflicting information. Because of its flash point trichloroethylene should be classified as a flammable liquid, but it does not behave like liquids with similar flashpoints, such as amyl acetate, amyl alcohol, butyl acetate, cyclohexanone, and cyclohexylamine.
Trichloroethylene is very difficult to ignite, even though it has a reported ignition temperature of 770°F. Its flammable range has been reported to be from 8 percent to 10.5 percent—a very narrow range that under normal circumstances would make the vapors difficult to ignite simply because it would be difficult to find them within the flammable range. However, some experimenters have shown the lower flammable limit to be as high as 12.5 percent to 14.5 percent, with the upper flammable limit also being reported as 18.4 percent and as astonishingly high as 90 percent. With a flammable range that can be interpreted (from all the data) as from 8 percent to 90 percent, a flash point of 90°F, and a very lowignition temperature of 770°F, trichloroethylene should be listed as a fairly hazardous flammable liquid, but it isn’t. One popular reference source lists trichloroethylene as nonflammable. Trichloroethylene, according to several sources, is very difficult to ignite, requiring a strong flame and high temperatures to do so. It has been used in great quantities for many years as a degreasing solvent for metals with a good safety record as far as fire is concerned.
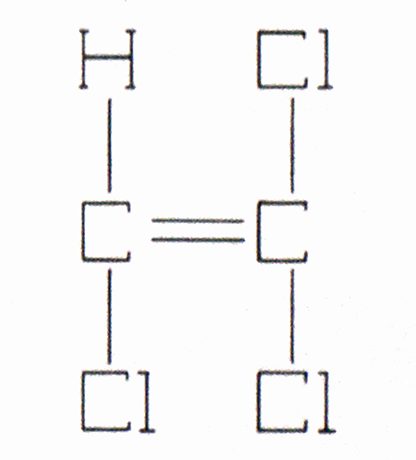
The liquid has a specific gravity of 1.46, a molecular weight of 131.4, and a vapor density of 4.53. It freezes at -99.4°F (some reports have it even lower), boils at 181°F, and is slightly soluble in water. Its chemical formula is C2HCI3 (sometimes written CHC1 = CCI2 and its structural formula is

HAZARDS
Even though trichloroethylene is reportedly not a fire hazard, it’s very dangerous to ignore the properties that say it will burn. No matter what difficulty is reported in the ignition of vapors of trichloroethylene, when the liquid reaches 90°F enough vapors are generated to produce an ignitable mixture; this ought to persuade anyone using the product (or in charge of safety when using it) to take the necessary precautions to eliminate ignition sources and keep the vapors from entering this flammable range. Although reports say that it takes “hot flame” to ignite trichloroethylene vapors, the relatively low ignition temperature of 770°F reported indicates that it does not take much energy to ignite the vapors. Conflicting reports of the product’s flammable range in itself ought to dictate that strict control of trichloroethylene vapors is required.
Manufacturers and users of trichloroethylene may object to the stringent controls suggested because, in reality, it has been difficult to ignite trichloroethylene vapors. However, it is always best to err on the side of conservatism, and controlling trichloroethylene vapors to prevent ignition will also lessen other hazards of the vapors.
If ignition does occur, the presence of chlorine in the molecule (which, incidentally, is the reason the material burns with difficulty) will produce combustion products in addition to those ordinarily generated by a hydrocarbon compound. These combustion products include hydrogen chloride, a corrosive gas that dissolves in moisture to form hydrochloric acid, and phosgene, a deadly poisonous gas.
Contact with trichloroethylene produces problems for anyone exposed. Occasional skin contact with the liquid causes mild irritation in most cases, but repeated contact causes roughness of the skin and possible infection.
Contact with the eyes can cause temporary burns, which usually heal well.
Ingestion causes irritation of the gastrointestinal tract, usually accompanied by nausea, vomiting, and diarrhea.
The major health hazard to exposed persons is the inhalation of vapors. Ordinarily these vapors irritate the eyes, nose, and throat, thereby warning potential victims of the exposure. However, some people may find these vapors pleasant smelling, which can cause serious problems. High concentrations of trichloroethylene vapors can cause depression to the central nervous system, clouding judgment. The victim may experience blurred vision, dizziness, fatigue, headache, nausea, unconsciousness, and even death.
In some cases people have found the vapors so pleasant that they have volunteered to clean up degreasing tanks of remaining trichloroethylene. Work rules usually include the use of selfcontained breathing apparatus and lifelines tied to workers to pull them from the tanks if they remove their masks, run out of air, or are otherwise overcome by the vapors. Deaths caused by overexposure to trichloroethylene vapors have come from asphyxiation, but those exposed have not noticed the effects of such deprivation of oxygen because of the vapors’ intoxicating effects.
There may even be evidence indicating that exposure to the vapors may produce a very mild addiction, causing individuals to expose themselves deliberately to the chemical. This can produce dangerous long-term effects on the liver and kidneys, as with chronic exposure to any halogenated hydrocarbon. Of course, short-term exposure may cause unconsciousness, which can result in death by asphyxiation.
The TLV-TWA (threshold limit value-time weighted average) for trichloroethylene is 50 ppm (parts per million of air). The STEL (short-term exposure limit) is 200 ppm and the IDLH (immediately dangerous to life and health) level is 1,000 ppm.
SYNONYMS
acetylene trichloride
Algylen
Anamenth
Benzinol
Blacosolv
Cecclene
1 -chloro-2,2-dichloroethylene
chloryea
chlorylen
Circosolv
1,1 -dichloro-2-chloroethylene
Dow-tri
Dukeron
ethinyl trichloride
ethylene trichloride
Fleck-flip
Fluate
Gemalglene
Germalgene
Lanadin
Lethurin
Narcogen
Narkosoid
NCI-C04546
Nialk
Perm-a-chlor
Petzinol trethylene
tri
Triad
Triasol
trichloran
trichloroethene
1.1.2-trichloroethylene Tri-clene
1.2.2-trichloroethylene Tri mar
Vestrol
Vitran and Westrosol
While trichloroethylene is considered a stable chemical, it reacts with a rather long list of chemicals, including metals such as the alkali and alkaline earth metals, aluminum and alloys, zinc and alloys, magnesium, and titanium. It also reacts with amines, azo and diazo compounds, cyanides, caustic materials, hydrazines, mercaptons, organic sulfides, mineral acids, peroxides, and oxygen under pressure. The reactivity may result in liberation of dangerous reaction products, or it may weaken such metals as aluminum, magnesium, titanium, zinc, and their alloys by the corrosive action it produces on contact with these metals.
NONFIRE SCENARIO
As with any release of a hazardous material, you must alert the proper environmental authorities. Since trichloroethylene usually does not produce a high degree of immediate risk to humans (other than asphyxiation and the chance of fire), incident commanders may have considerably more time to consider mitigation suggestions from environmental authorities than if a much more dangerous material were involved.
Since trichloroethylene is a somewhat volatile liquid, any release of the product produces large amounts of vapor. These vapors “hang together” because of the high vapor density and flow along low spots in the terrain. There is a tendency for all vapors with high vapor densities to gather in low places or confined areas, posing a great hazard for anyone caught in such a concentration without respiratory protection. These “clouds” and other accumulations of vapor may be dispersed with a highpressure fog or spray. All water used in this manner must be confined.
Keep in mind that although reported ignition of trichloroethylene vapors is rare, the possibility does exist that ignition will occur. Therefore, treat trichloroethylene as a flammable liquid.
If die container involved in the release is leaking (rather than having failed catastrophically), attempts should be made to capture the leaking liquid in a secure container. In some instances, containers may be used to temporarily collect the liquid, which can then be transferred to a secure container. On some rare occasions, it may be possible to pump the collected liquid back into the leaking container until a secure container can be procured. This will not be a closed system, and vapors will be generated. In any event, only compatible materials should be used in handling, pumping, storing, or any contact with the product.
IDENTIFICATION NUMBERS AND RATINGS
CAS
(Chemical Abstract Services)
79-01-6
STCC
(Standard Transportation Commodity Code)
4941171
RTECS
(Registry of Toxic Effects of Chemical Substances)
KX4550000
UN/NA
(United Nations/North America)
1710
RCRA
(Resource Conservation and Recovery Act)
U228
CHRIS
(Chemical Hazard Response Information System) TCL
DOT
(U.S. Department of Transportation)
ORM-A
NFPA 704 Rating
(National Fire Protection Association)
2-1-0
IMO
(International Maritime Organization)
6.1, poisonous substance
Trichloroethylene liquid must be prevented from entering sewer systems and waterways, as well as from spreading on land. Containment ponds may be quickly constructed by pushing soil, sand, clay, or other materials into dikes surrounding the spill. A containment pit may be dug and trenches dug to lead the liquid to the pit. Once contained, the liquid may be salvaged by the shipper, the manufacturer, or the buyer of the product. The environmental authorities should supervise the salvage of the product and determine the extent to which contaminated soil must be removed.
Firefighting foam may be used to control the evolution of vapors from the spill. Sheets of compatible material may also be used to cover the contained liquid. Preparation must be made to reinforce the dikes or make them higher in the event of rain. In fact, covering the trichloroethylene with a layer of water very effectively controls the evolution of vapors as long as the containment pond or pit is large enough to contain the added volume. Since trichloroethylene is heavier than water and only very slightly soluble in water, this technique is used in degreasing tanks to control vaporization of the product.
If the spill is large, the generation of vapors will be large (except in cold weather). Consider evacuation downwind early in the incident.
Once the product has been removed by professional cleanup crews, any remaining product may be absorbed with cement powder, clay, fly ash, sand, soil, or any other absorbent material. This material must then be disposed of under the supervision of environmental authorities in accordance with federal, state, and local regulations.
GLOSSARY
Aeration—a process by which air is introduced into a liquid, either by bubbling the air through it or spraying the liquid into the air at normal pressures. Also called sparging or air stripping, which is the spraying of the liquid or gas into the air to remove the contaminant.
Alkali metal—a member of Group I on the Periodic Table of the Elements: lithium, sodium, potassium, rubidium, cesium, and francium. Alkaline earth metal—a member of Group II on the Periodic Table of the Elements: beryllium, magnesium, calcium, strontium, barium, and radium.
Caustic—any strongly alkaline substance that has a corrosive effect on tissue; usually refers to bases. Flammable range—the concentration of gas or vapor in air between the lower and upper flammable limits. It is the range of concentrations in air in which ignition will occur. Flash point—the minimum temperature of the liquid at which it produces vapors sufficient to form an ignitable mixture with the air at the surface of the liquid or container. Be careful of published flash points because of die possibility of different values from using different methods. Check several references and use the lowest value reported.
Ignition temperature—the minimum temperature to which a material must be raised before it will ignite. Lower flammable limit—the minimum percentage of gas or vapor in air below which ignition will not occur (the mixture is too lean). Also called the lower explosive limit (LEL).
ORM-A—the DOT designation for “Other Regulated Material.” The “A” designation is for materials that have an anesthetic, irritating, noxious, toxic, or other similar property that can cause extreme annoyance or discomfort to passengers or crew if a leak occurs during transportation. Upper flammable limit—the maximum percentage of gas or vapor in air above which ignition will not occur (the mixture is too rich). Also called the upper explosive limit (UEL).
Volatile—the capability of evaporating rapidly at normal temperatures and pressures.
If the liquid enters a sewer system, notify the local sewage treatment facility immediately. The sewer system will probably fill with vapors, which may be forced back into an occupancy, so you must alert the community.
If trichloroethylene enters a waterway, alert all downstream users immediately. Intake into drinking water systems or industrial plants can produce dangerous results. As the liquid enters a waterway, it will immediately sink to the bottom. If the waterway is slowmoving, the liquid will collect in low spots and may be vacuumed into secure containers. If the waterway is fast-moving, trichloroethylene will flow along the bottom. Damming the waterway may allow the liquid to accumulate against the dam, and it can then be removed. Diversion of the waterway into adjacent low-lying areas can confine the product but may contaminate the collection area. Contaminated water may be purified by aeration techniques.
Trichloroethylene may be removed from water by adding certain types of clay, limestone, peat moss, or activated carbon. The product will be adsorbed by the additive and can be removed under supervision. A spill that is confined near a waterway can generate vapors that flow over the surface of the stream or river, which can cause a problem for anyone in boats or on the shore downstream of the spill.
FIRE SCENARIO
Any exposure of a container of trichloroethylene to radiated heat or flames from a fire can cause dangerous pressure rises within the container, which could lead to explosive failure of the container. Cooling -water should be applied to exposed containers from safe distances, perhaps by unmanned monitors.
Trichloroethylene will burn, and therefore the probability of its vapors being ignited by a surrounding fire is high. Again, if this product is treated as other flammable liquids are in a fire (or even a nonfire) situation, the odds of successful incident mitigation are in favor of the emergency responders.
Burning trichloroethylene may be extinguished with water fog or spray, carbon dioxide and dry chemical (depending on the size of the fire and application conditions), or foam. A burning pool of trichloroethylene may be extinguished by gently applying a layer of water over its surface, assuming the volume of added water can be contained by the container or containment pond or pit.
Burning trichloroethylene will generate large amounts of toxic combustion products such as carbon monoxide and phosgene. It also will generate irritating vapors of hydrogen chloride. If you have not evacuated downwind of a spill, you must do so if fire is threatening or has started. All emergency responders in this situation must have respiratory protection in the form of positive-pressure, self-contained breathing apparatus.
FIRST AID
For inhalation of trichloroethylene, move the victim to fresh air and keep quiet and warm. Administer artificial respiration only if breathing has stopped or become difficult, being careful of exposure to the product in the victim’s lungs and vomit. Do not administer epinephrine (adrenalin). Seek medical attention immediately.
If the victim has ingested trichloroethylene and is conscious, there are different opinions as to whether the victim should be induced to vomit. Let , medical personnel make that decision.
For eye contact with trichloroethylene, flush the eyes immediately with water for 15 minutes, occasionally opening the eyelids. Medical attention is also necessary.
For skin contact, remove all contaminated clothing immediately. Wash all contacted body areas with large amounts of soap and water and seek medical attention.
PROTECTIVE CLOTHING AND EQUIPMENT
Choose clothing and protective equipment to prevent contact of trichloroethylene liquid or vapor with the eyes, skin, or respiratory system. Select splashproof chemical goggles and face shield for eye protection and use positive-pressure, self-contained breathing apparatus, especially if high vapor concentrations of decomposed product may be present. Rubber boots, gloves, and aprons will offer some protection, but wear total encapsulating suits if contact with trichloroethylene is possible. Manufacturers of such protective equipment claim that suits made of chlorobutyl rubber, nitrile-butadiene rubber (NBR), and Viton may offer protection.

