Plastics and Fire Investigations
FEATURES
ARSON
It seems that every time the subject of plastics arises, especially among firefighters, the discussion always turns to the hazards, either real or imagined, of burning plastics.
There can be no denying that plastics burn, just as there can be no argument that burning plastics will produce toxic combustion products, as do all organic materials whether natural or manmade (see “Plastics—Some Facts Firefighters Should Know,” FIRE ENGINEERING, February 1984). If the firefighter would take the same precaution in approaching burning plastics as he does in approaching any burning material, namely wearing his self-contained breathing apparatus (SCBA), he would be in no more danger than if the fire room contained only “traditional” materials (wood, paper, glass, metal, cotton, etc.). However, if the firefighter pays very close attention to the fire room, he can better learn how to advance his understanding of just what occurred in the fire; and the clues will come from a very surprising source—plastics!
The simple fact of the matter is that plastics have permeated our lives to such an extent that it would have to be an extremely unusual room that contained none. The reasons for this are many and varied, but the bottom line is that plastics are wanted and needed by society. They perform the jobs they are asked to do or they wouldn’t be used at all. You and I and other consumers demand them, so they find their way into all facets of our lives. This is how they have found their way into every room in our homes and the rooms of most buildings. Because they are where they are, fire investigators can profit from their presence.
The problem has been that firefighters have had such strong negative feelings about plastics that they have not thought that plastics could be of help in fire investigations. With the myriad of plastic articles representing a wide variety of shapes, sizes, and uses, knowing just a few simple physical properties can help the fire investigator determine with some fair degree of precision just what happened during the fire. In many cases, the observation of destroyed or partially destroyed plastic articles can fill in the missing clues that the investigator has been looking for.
REVIEW OF PLASTICS
Before we look at how fire investigators can use plastics to give them clues as to what happened in a particular fire, we must first go over a quick review of what plastics are.
Plastics belong to a huge group of materials called polymers. Polymers are nothing more than very long-chain molecules made up of thousands and thousands of much smaller molecules known as monomers. The process (in a giant vat or reactor) of converting monomers to polymers is a chemical reaction known as polymerization.
Polymers exist in two large classes, natural and synthetic (man-made). Mother Nature is the greatest polymerizer of all, and the most common natural polymers are cotton, wood, paper (made up principally of cellulose), silk, wool, and leather. The man-made polymers include plastics and rubber. “Plastics are further broken down into thermoplastics and thermosets. Thermoplastics are those plastics that may be processed (that is, heated and subjected to pressure) more than once, while thermosets may be processed only once. Thermoplastics may be ground up and recycled through the processing equipment several times successfully, while thermosetting plastics will begin to char when they are subjected to heat a second time.
The thermoplastics are more familiar to most people because their use is more widespread than the thermosets. The most common thermoplastics and their monomers (in parentheses) are:
- Polyethylene (ethylene)
- Polyvinyl chloride (vinyl chloride)
- Polystyrene (styrene)
- Polypropylene (propylene)
- ABS (acrylonitrile-butadiene-styrene).
Some thermoplastics like polyethylene terephthalate and thermoplastic polyurethane are polymerized from materials that are not monomers in the truest sense, but end up as thermoplastics anyway.
Thermosetting plastics are not as familiar to most people as the thermoplastics because they are more difficult to process and are not as versatile as the thermoplastics. The most common thermosets are urea-formaldehyde, melamines, epoxies, and phenolics. temperature of a plastic is that point at which the material begins to flow. This may be more accurately described as the fluxing temperature, and for the purpose of this discussion the term melting temperature will mean that temperature at which the plastic begins to flow rather than turn to a liquid.
Continued on page 50
Continued from page 46
Each plastic, whether it’s a thermoplastic or thermoset, is a totally different material, and therefore has different physical and chemical properties. A few are related to each other chemically, like polyethylene and polypropylene, so their properties may be somewhat similar. However, through compounding [which is the addition of all sorts of organic and inorganic materials (even other plastics) to the original polymer to deliberately give it different properties] even the same polymer may be made to behave like something totally different. Polyvinyl chloride, or PVC as it is commonly known, is a perfect example.
PVC can be made as a very rigid material like house siding or pipe, or a very soft, pliable material like automobile upholstery. It can be made to be weatherable or to degrade in the sunlight. It can be made into a container to hold corrosive materials, or it can be made to safely package food. There are literally thousands of other possibilities, each slightly or vastly different in its behavior (in a fire or otherwise) than the other.
Another consideration is the use to which the plastic is put; that is, its size, shape, configuration and whether it’s free-standing, laminated to, or covering something else. The soft, pliable PVC used in upholstery (whether in an automobile or over furniture) will behave differently in a fire than the soft pliable PVC that is coated onto cloth and used as a wall covering. Also, a very thin piece of plastic will ignite and burn very differently than a large, thick piece of the very same polymer compounded identically.
IMPORTANT PROPERTIES AS CLUES
The fire investigator can add to the clues given to him by other materials by paying attention to the condition of plastic articles in a fire room. Knowing additional properties of plastics can help the fire investigator refine the information he needs to gather about a fire.
The ignition temperature of a plastic article exposed to a fire would seem to be the most important property under consideration, but that may not always be the case. If a plastic article does ignite and is totally consumed in the fire, the only thing you can be sure of is that the heat generated by the fire was sufficient to raise the temperature of the article high enough to cause ignition.
The investigator must also realize that the range of ignition temperatures of most common free-standing plastic articles lies well within the temperature range generated by almost all fires. Clues are available to the investigator if the plastic article is ignited by radiant heat from a fire that is some distance from the article in question and perhaps moving away from the plastic. He can then determine the intensity of the fire by estimating how much energy would have had to be released to cause ignition of the plastic article.
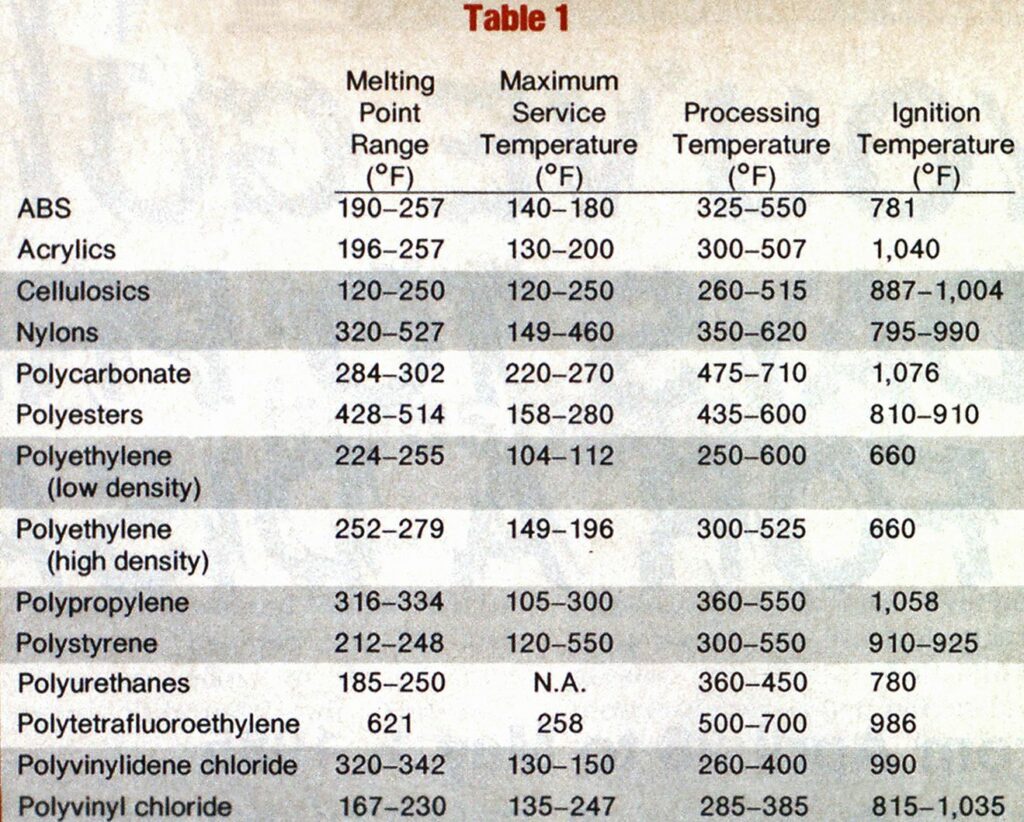
Temperatures
The statement that the ignition temperature might not be the most important property should have been a tip-off to you that there are other properties of plastics that you should be aware of. At least three other temperatures are important; service temperature, melting temperature, and processing temperature.
It is probably a good idea to stop here and define these four temperatures so that their differences can be highlighted.
The ignition temperature of any material is the minimum temperature to which that material, in air, must be raised before self-sustained combustion will occur (some plastics, like rigid PVC, will not sustain their own combustion).
The service temperature of a material is the maximum temperature to which the material can be raised and still perform the function for which it was designed. There are many materials that are weakened by exposure to heat and, therefore, are no longer able to perform whatever function the engineer had in mind for them. Steel beams, which have twisted after exposure to a fire, are examples of a non-plastic material being exposed to temperatures above their service temperature.
The melting temperature of a material is that temperature at which the material changes from a solid to a liquid. The melting
Continued on page 52

Consulting tables listing key temperatures and typical burning characteristics of common plastics are but a starting place for the fire investigator as he begins to use plastics to help “fingerprint” a fire. He must learn what the most common uses of the most common plastics are. He must commit to memory the different plastics that might be used to make the same article. There are several articles whose composition may always be the same plastic or even the same compound; but it is more likely that some common plastic articles most often used in homes are made by different manufacturers and from different plastic materials.
The processing temperature of a plastic is the temperature at which the plastic will be very fluid, but not necessarily a liquid. In other words, when a plastic material is processed (subjected to heat and/or pressure), it assumes a dough-like consistency so that it can be easily pushed through a die or into a mold. The processing temperature will be higher than the reported melting temperature.
The manifestations of these four temperatures, or, more correctly, temperature ranges, might not be obvious, so let’s examine them.
The first, ignition temperature, is the easiest to see and understand. If there is evidence that the plastic article did indeed burn, you can be sure that its ignition temperature was reached, if the plastic was not totally consumed in the fire, and its burning was not extinguished by firefighters, assumptions can be made about the course of the fire, the restriction of oxygen, and other assumptions that the investigator would ordinarily make when viewing a fire room after extinguishment. Additionally, there are some plastics that will not burn very easily. Knowing the oxygen index (a measurement of how much oxygen is needed to support combustion of a material) of each plastic will pinpoint the restriction of oxygen.
The service temperature is as important to the firefighter as it is to the fire investigator. The firefighter should become familiar with the service temperatures of all plastics used in building construction, room design, etc., because failure at a crucial time while the firefighter may be in or near the building could be harmful. For example, unwanted and unexpected ventilation could occur when plastic glazing fails, resulting in serious problems such as localized backdraft, flashover, or rollover within the fire occupancy.
If the fire investigator becomes familiar with these service temperatures, valuable clues will be available to him. If the plastic part fails (usually by sagging or otherwise losing its strength and integrity) but doesn’t burn or melt, the investigator can pinpoint with some degree of accuracy the maximum temperature reached near the part. In some cases he might be able to tell how long the part was exposed to that heat because some service temperatures have times attached to them (for example, 300°F for 30 minutes). With the great proliferation of plastic structural parts and engineering uses, combined with the myriad of other uses for every room in the average house, the fire investigator should begin to see the many possibilities of “fingerprinting” the fire using plastics.
The melting temperature, or fluxing temperature, will give another set of clues to the investigator. All plastic parts and articles exposed to the heat of a fire will have some weakening of their chemical and physical bonds and begin to soften. Further absorption of heat energy will cause the part to sag, and then flow under the influence of gravity. For each plastic, there is a range of temperatures within which this will occur. If the article is freestanding, temperature ranges instead of specific temperatures are given because each manufacturer may make his polymer molecule different from others, or a compounder adds ingredients that will alter some properties at different temperatures. If the plastic is coated onto, or covering something, or is in contact with anything that will act as a heat sink, the temperature range will obviously be different. If the investigator, for example, finds the empty plastic milk container partially melted, or the wall telephone stretched and distorted as it sags down the wall, all he needs to do is look up the melting temperature of polyethylene (for the milk bottle) and ABS (for the telephone) and he will be able to tell the temperatures that these articles were exposed to.
Table 2 Burning Characteristics of Common Plastics
ABS plastics burn with the characteristics of hydrocarbon plastics (plastics that contain only carbon and hydrogen in their molecules, like polybutadiene, polyethylene, polypropylene, polybutylene, and polystyrene), since two of its polymers are polybutadiene and polystyrene. It will sag and burn with a fairly dark, sooty flame, emitting large chunks of soot.
Acrylics burn somewhat slower than hydrocarbon plastics because of the presence of oxygen in its molecule. (Other plastics containing carbon, hydrogen, and oxygen in their molecules are acetal, cellulosics, ethylene vinyl acetate, polycarbonate, and polyesters.) They will burn with a blue flame with a yellow tip and have a fruity smell.
Cellulosics bum with a blue or yellow flame, may emit small sparks, and the melted drops may burn. They smell like vinegar or rancid butter.
Nylons will burn with a blue flame and emit an acrid smell. The drippings will be burning.
Polycarbonate burns with difficulty and leaves a char. It has a smell that’s unique to polycarbonate.
Polyesters burn much like the acrylics. The thinner parts will sag and “back away” from heat.
Polyethylene burns with a yellow-tipped blue flame with the drippings burning freely. The odor emitted is of candlewax.
Polypropylene burns very much like polyethylene but the odor might be sweeter.
Polystyrene burns with a yellow flame and emits characteristic large clumps of soot. The odor will be sweet.
Polyurethanes burn with a yellow flame with a blue base and emit an acrid odor.
Polytetrafluoroethylene (Teflon) is very difficult to burn, but if it does, it burns with a yellow flame.
Polyvinylidene chloride is difficult to burn, but when it does, it burns with a yellow flame with a green base, emitting heavy smoke and a pungent odor.
Polyvinyl chloride is difficult to ignite and maintain ignition, but when it burns, it does so with a yellow flame with green edges, white smoke, and a pungent odor.
Any time the investigator finds a plastic article totally melted, as the plastic milk bottle might do, leaving a puddle of what looks like candle wax, he can refer to the processing temperatures of the plastic in question. It is in processing the plastic resin or compound that the highest temperatures are reached. Again, knowing what plastic is used in making the part or article that has melted, the investigator needs only to look up the processing temperature (or temperature range) of that plastic. This time he will find the minimum temperature that the part was subjected to.
A word of warning when investigating a fire in a room or area containing certain plastics having low melting temperatures: Some plastics, like polyethylene and polypropylene will drip and flow rather easily, burning as they flow. Don’t let clues left behind by these flowing, burning materials lead you to conclude that combustible liquids might have been used. Knowing the burning characteristics of these and other plastics may actually help you in determining cause and origin, rather than confusing you.
Finally, a word about temperature ranges. Earlier, we said that the differences among plastics is due to the chemical makeup of the monomers used in polymerizing the plastics. The manner in which they were polymerized (the amount of heat and pressure to which they were subjected and their physical form during the process) accounts for some more property differences; and the addition of flame retardant chemicals to plastic resins and compounds will alter their burning characteristics radically. Whatever is added to the plastic to impart other properties will ultimately affect these four important temperatures. Since no one can know with any degree of certainty what the exact makeup of the plastic used in making a particular article is without talking to the resin manufacturer, compounder, or processor, it makes more sense to provide a range of temperatures within which it is expected that nearly all plastic articles will be covered.
BURNING CHARACTERISTICS OF COMMON PLASTICS
Many plastics can be identified by the way they burn. A fire investigator, who is always involved after a fire, may wonder how he is going to be helped by knowing how a plastic burns. First, some plastics will leave telltale residues or other clues to tell the investigator what burned. Second, he may not know what particular plastic was used in manufacturing a certain article that was partially consumed in the fire, but he may be able to determine what the plastic was by deliberately burning a sample left by the fire. Since most plastics burn in a distinctive manner, he may be able to do a little qualitative analysis on his own.
Some things, however, occur because of shape rather than the type of plastic used in making the article. For instance, plastic bottles (mostly one gallon milk bottles), if empty when the fire approaches and not destroyed by the fire, will melt, starting at the end or side facing the heat, thereby “pointing” at the fire or source of heat. If the plastic article is thin enough, like a film application, the thin piece of plastic will “back away” from the heat, thereby producing another clue that will indicate the direction of the fire or heat source. Neither of these actions may seem earthshaking now, but one never knows when the simplest of clues may help solve a seemingly impossible mystery.
Table 3
Composition of Common Plastic Articles


PLASTICS THAT DO NOT BURN EASILY
Polyvinyl chloride (PVC) is the classic example of a plastic that will not burn very easily. As a matter of fact, it should be of paramount importance for every fire investigator to know that PVC cannot be a source of ignition because of its molecular makeup. Vinyl chloride, the monomer of PVC, contains chlorine in its molecule. If an ignition source is applied to an article made of rigid PVC, the polymer begins to decompose (resembling pyrolysis, the breakdown by heat). As it decomposes, the chlorine in the molecule acts as a very efficient freeradical quencher, and if the ignition source is removed, any fire begun will go out. It is also important here to note that no free chlorine is ever liberated by burning or decomposing PVC. The combustion products of PVC include water, carbon dioxide, carbon monoxide, and hydrogen chloride, an irritant that forms hydrochloric acid when dissolved in water. But there is never any free chlorine liberated by PVC.
The important thing to note is the difficulty with which PVC burns. If the investigator discovers that a substantial amount of PVC has burned, he can safely assume that a hot supporting fire of some other material caused the PVC to burn. Some experimentation by the investigator will indicate to him the size, intensity, and energy output of a fire to actually consume rigid PVC. Rigid PVC can neither be an ignition source nor a fire-sustaining material.
There are many uses of semi-rigid and flexible PVC. In this case, a plasticizer, an organic material used to soften rigid PVC, is added. The presence of a plasticizer lowers the oxygen index of PVC, but not by very much. Semi-rigid PVC still will neither burn very easily nor sustain combustion. Only very soft and pliable PVC has a compound that will sustain combustion, and then usually only in a very thin configuration and hanging in a vertical position.
In addition to PVC, the plastics polyvinylidine chloride, chlorinated polyvinyl chloride, and chlorinated polyethylene contain halogens (the name for the family of elements containing fluorine, chlorine, bromine, or iodine). Like vinyl, none of these materials burn as easily as traditional materials. The fluorinated polymers too, including polytetrafluoroethylene (Teflon), are essentially non-burning plastics, again because of the presence of the halogen in the molecule.
If a polymer has had a flame retarding ingredient added to it, it will usually pass very stringent tests for combustibility. The implication here is that many polymers that are considered to burn readily may be compounded in a manner that they can be considered plastics that don’t burn easily. Many household appliances, like television sets, must have very fire resistant plastic shells. If parts made of these materials do burn, you can again be sure that there was a very hot fire of some other material responsible for the burning of the plastic part. The investigator must become familiar with the uses of specially compounded plastics.
SUMMARY
By themselves, the properties of particular plastics may not seem as though they would be a tremendous help to investigators, but my purpose is simply to add to the investigator’s repertoire of knowledge. He already gathers clues by observing the appearance of traditional materials once the fire is extinguished. I now propose that he begin looking more closely at articles made of plastic, so that he can better determine what actually happened.
It is not enough that the investigator knows that polyethylene burns with a dripping flame and that it might flow and burn, leaving either distinctive burn patterns or patterns that might lead him to an incorrect conclusion. He should know that although many parts and articles are made of polyethylene, their shape and thickness may cause them to behave differently in the same fire. Indeed, different articles of the same plastic in the same room exposed to the same heat source could provide invaluable information as to the progress the fire made by melting or burning in different patterns. The investigator should also be aware that while one manufacturer always manufactures an article out of high density polyethylene, another may use polypropylene, and a third may use PVC.
Of course, the fire may have been so intense that the entire room was destroyed. In that case, plastics used in the destroyed room will be of little help to the investigation. But don’t forget to look at plastic articles in adjoining rooms or in cupboards and cabinets. If these articles melt and/or flow in a particular manner, an unexpected clue might be available.
Continued on page 56
Table 4
Reference Texts on Plastics’ Uses and Properties
The Modern Plastics Encyclopedia, McGraw-Hill Inc., 1221 Avenue of the Americas, New York, NY 10020 (published annually).
Plastics Technology Manufacturing Handbook and Buyers Guide, Bill Communications, 633 Third Avenue, New York, NY 10017.
Flammability for Plastics, Carlos J. Hilado, Technomic Publishing Co., 851 New Holland Avenue, Lancaster, PA 17614.
Combustion Toxicology, Principles and Test Methods, Kaplan, Grand, and Hartzell, Technomic Publishing Co.
Fire and Flammability Series, Volume Thirteen, Fifteen, and Seventeen, edited by Carlos J. Hilado, Technomic Publishing Co.
Fire Safety Aspects of Polymeric Materials, Volumes One through Ten, National Materials Advisory Board, Technomic Publishing Co.
Behavior of Polymeric Materials in Fire, edited by E. L. Schaffer, American Society for Testing and Materials, 1916 Race Street, Philadelphia, PA 19103.
Facts and Figures of the U S. Plastics Industry, The Society of the Plastics Industry, 355 Lexington Avenue, New York, NY 10017.
Kline Guide to the Plastics Industry, Kline Publications, 330 Passaic Avenue, Fairfield, NJ 07006.
Continued from page 54
The very fact that so many uses have been found for plastics will assure that there will be many different articles made of many different plastics in most homes and offices. Each one of these articles will react differently to the same fire, and it is this difference in behavior that will help produce “fingerprints.”
In a typical room, the fire investigator may be able to use the ignition temperature of a plastic used in one article that burned (or didn’t burn), the service temperature of a structural part that failed (or didn’t fail), the melting point of an article that deformed and sagged (or didn’t), and the processing temperature of an article that really sagged and flowed. As these articles and parts are probably in different parts of the room, a picture can be visualized as to the heat patterns produced. As a matter of fact, even if the articles are close to each other in the room, the application of behavior at certain temperatures will tell the alert investigator a great deal.
If one remembers that shape, size, configuration, nearness to other materials that might act as heat sinks, and the addition of compounding ingredients all affect the four important temperatures provided for each plastic, experience and some experimentation will lead the fire investigator to become an expert on the uses of plastics and how those plastics react to heat.
There is no simple or magical way to use plastics in the solution of fire origin or cause. It takes the same hard work and the same accumulation of experience that led the fire investigator to become an expert in the first place. But with a little common sense and a lot of perserverence, he can add a very valuable tool to those he possesses now.


