Don’t take your work problems to your home and don’t take your home problems to work is sound advice. At one fire department, firefighters voiced their concerns to the infection control officer about what they might be exposed to at work that they might take home and expose their families to. How can firefighters reduce or prevent their exposure to and the spread of communicable infections? Reflecting on the previous year’s annual infection control education, members wondered whether they were doing enough for infection control. They had concentrated on the big items (e.g., cancer reduction, injury prevention, and physical/mental health), but did they miss something?
Background and Problem Identification
According to nationally recognized standards such as National Fire Protection Association (NFPA) 1581, Standard on Fire Department Infection Control Program, and regulations such as blood borne pathogen standard Occupational Health and Safety Administration (OSHA) 1910.1030, training on communicable diseases is required upon hire and annually. Generally, most training focuses on bloodborne pathogens and other communicable diseases and how to prevent exposure. Although training on this topic is vital to protecting the health of firefighters and other first responders, it is often something completed as part of a checklist.
RELATED FIREFIGHTER TRAINING
Duties of a Designated Infection Control Officer
The Designated Officer’s Role for Infection Control
Hands On Infection Control – The Flu
Departmental policies and procedures also assist in identifying the minimum amount of personal protective equipment (PPE) to wear according to incident type and risk management considerations. We protect ourselves from infectious agents by donning proper PPE prior to potential exposure. However, what if the PPE we depend on is also a pathway for exposure to infectious disease?
Not all our protective equipment is disposable or personal. For example, our self-contained breathing apparatus (SCBA) is a shared piece of safety equipment that annual infection control education historically might not have considered.
Previous studies have indicated that S. aureus is all too commonly found in fire stations. A Tucson, Arizona, study found that of nine stations tested, 20 percent of the station couches and desks tested positive for S. aureus; 67 percent of those isolates were specifically methicillin-resistant Staphylococcus aureus (MRSA).1 The Tucson study sample sites included lockers, couches, remote controls, and offices, focusing on items that several people would regularly come in direct contact with. An August 2012 report noted that readily available research covering MRSA and the fire service is not abundant.2
Based on the Centers for Disease Control and Prevention (CDC) claim that approximately one-third of people carry S. aureus in their nasal passages,3 it is hypothesized that at least 30 percent of fire department employees are colonized with S. aureus. It may be even higher; other studies suggest that those with patient contact—e.g., health care workers and first responders—are more likely to be S. aureus carriers.4 Therefore, it is reasonable to speculate that shared equipment, especially our SCBA, could be a reservoir for bacterial pathogens such as S. aureus. S. aureus is linked to a number of infections, ranging from mild skin infections to life-threatening wound and toxin-mediated infections. MRSA strains of S. aureus have become especially problematic in recent years because of their antibiotic resistance, making these infections incredibly challenging to treat. Those with weakened immune systems, the very young, and the very old are particularly susceptible to severe S. aureus infections.
Since S. aureus nasal passage colonization is a common finding, and it is not uncommon for fire departments to share SCBA equipment or components such as a face piece regulator with fellow firefighters, we decided to further investigate SCBA components as a potential source of exposure. In conjunction with the Texas Tech University Health Sciences Center in Lubbock, Texas, we performed an epidemiologic survey assessing the presence and prevalence of S. aureus and, more specifically, MRSA on and within SCBA surfaces worn by firefighters.
Study Design and Results
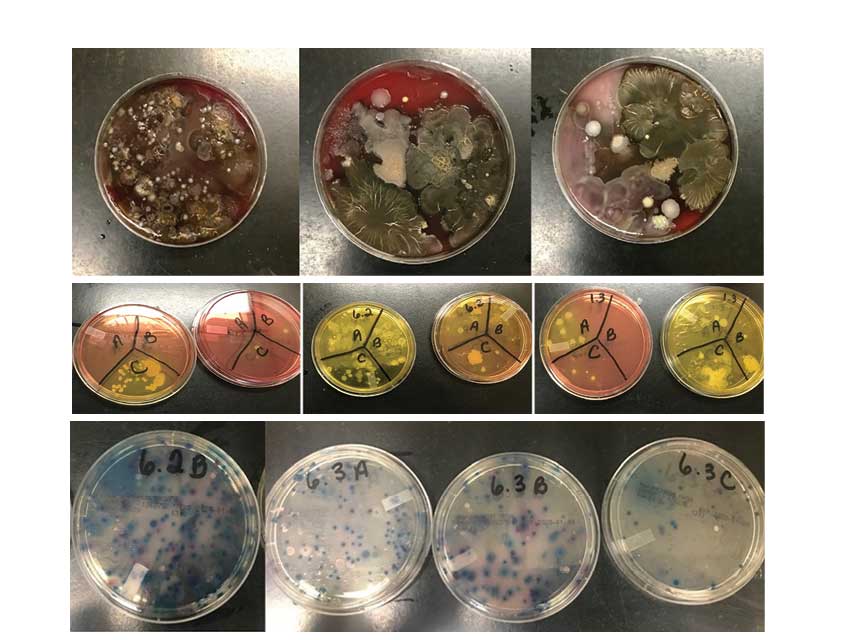
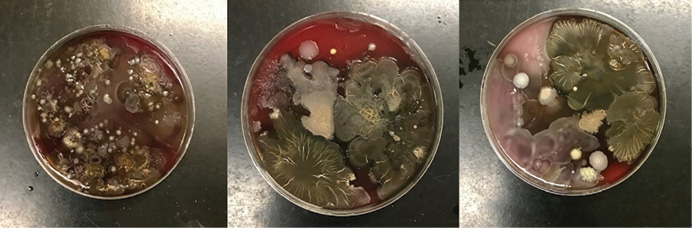
We obtained samples from 30 apparatus-assigned SCBA across 10 engine companies within the same organization, which accounts for roughly 25 percent of the participating fire department’s front line SCBA. We selected three SCBA sites to assess the presence of S. aureus and MRSA contamination: the SCBA face piece regulator opening, inside the face piece regulator assembly, and the waist strap harness that secures the regulator to the SCBA when not in use (photo 1). We chose these sites secondary to the locations that nasal secretions can potentially come in direct contact with during SCBA use. Using standard precautions, we swabbed each site in triplicate and plated on growth agar plates (a commonly used solidifying agent) that allowed the bacteria to grow and allowed us to differentiate between harmless bacterial species and the potentially pathogenic. Using agar that supported all types of microbial growth, we first looked at all that was living on the SCBA (photos 2-4). We also swabbed unboxed and unused SCBA as a negative control; no microbial growth was noted. We then used agar plates that allowed us to detect S. aureus and closely related species specifically (photos 5-7). We found that out of the 30 SCBA sampled, all 30 (100 percent) contained some species of Staphylococcus. Staphylococcus epidermidis was present on almost all SCBA. This microbe is commonly found on the skin and is generally nonpathogenic. We discovered that 72 out of the total 90 samples obtained were positive for the presence of S. aureus, 80 percent of all sample sites. Once we identified apparatus-assigned SCBA that contained S. aureus (Figure 1), we determined the percentage of S. aureus that were MRSA strains. Using agar plates containing antibiotics, 28 out of the 30 SCBA had probable antibiotic-resistant (ABX-resistant) S. aureus, which accounts for 93 percent of the SCBA that were sampled (Table 1). We then sampled those same sites using the more specific ChromAgar® plates for colorimetric detection of MRSA strains (photos 8-9 and Figure 2). MRSA was detected on 23 of 35 sites, accounting for 65.71 percent of the total sample sites. Overall, 53 percent of the SCBA had MRSA in one or more of the sample locations (Table 1). Surprisingly, out of the three sample sites indicated for each SCBA, it appeared that the most commonly contaminated site was the waist-strap regulator harness (sample site C) (Figures 1-2).

(1) Using sterile technique, each SCBA sample site indicated was swabbed and plated on various agar plates and incubated to allow for microbial growth: site A, regulator entry; site B, inside regulator; and site C, the regulator harness. (Photo by Shane Parker.)
(2-4) Representative microbial growth on blood agar (BA) plates. BA is a generic agar used to support the growth of a wide variety of microbial species. Using sterile technique, SCBA were swabbed, plated on BA plates, and incubated at 37°C for 24 hours. After 24 hours of growth, multiple species of bacteria and fungi were detected as a result of swab samples taken from the SCBA. (Photos 2-4 by Cassie Kruczek.)
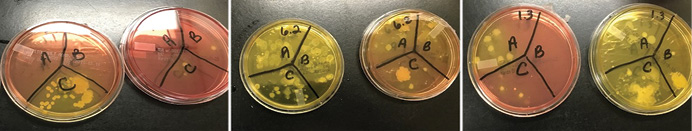
(5-7) Representative Staphylococcal growth on mannitol salt agar (MSA) plates (left-side images) and MSA containing the antibiotic oxacillin (right-side images). MSA is a selective and differential media, selecting for salt-tolerant bacterial species; it also contains color-change indicators to allow for differentiation between different Staphylococcal species. Bacterial growth in areas appearing yellow indicate the presence of S. aureus. Growth on MSA plus oxacillin indicates the presence of antibiotic-resistant bacterial species. (Photos by Cassie Kruczek.)
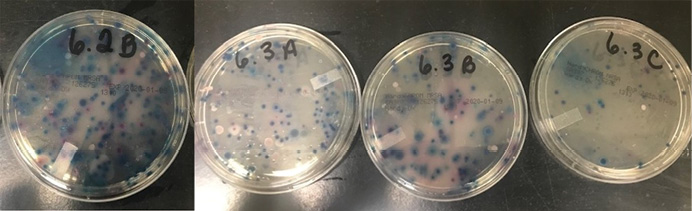
(8-9) Representative antibiotic-resistant Staphylococcal growth on HardyCHROM™ agar plates from sample sites. Using colorimetric indicators and antibiotics that inhibit the growth of methicillin sensitive S. aureus, HardyCHROM™ agar is used for the identification of MRSA and other methicillin-resistant Staphylococcal species. Gray-to-blue colonies represent methicillin-resistant S. intermedius, while purple-magenta colonies represent MRSA. (Photos by Cassie Kruczek.)
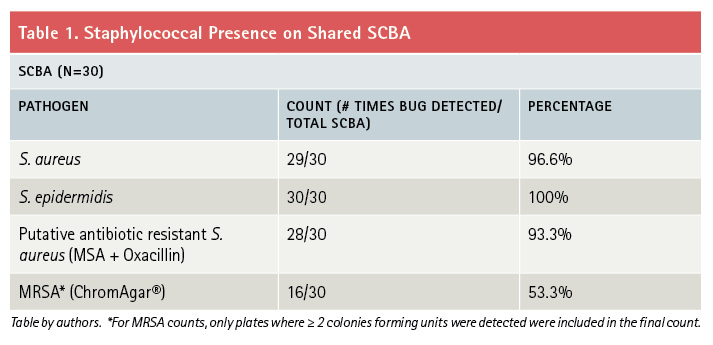
Figure 1. S. Aureus Presence by Sample Site
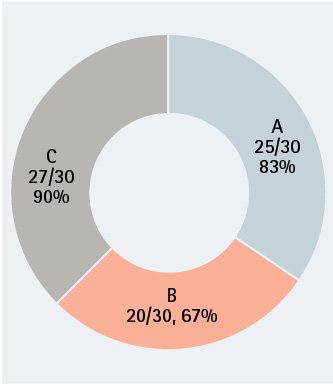
Scoring of S. aureus by SCBA sample site. Of 30 SCBA tested, most had S. aureus contamination. The highest bacterial load was located on sample site C, the regulator strap harness. (Figures by authors.)
Figure 2. MRSA Presence by Sample Site
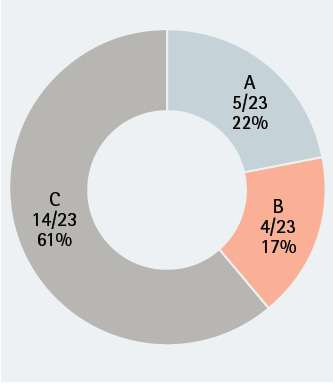
Scoring of MRSA by SCBA sample site. Of 23 SCBA tested, many had MRSA contamination. As was seen with S. aureus contamination, the highest bacterial load was located on sample site C, the regulator strap harness.
Where Do We Go from Here?
This study provided compelling evidence that we need to implement contamination reduction practices to safeguard our fire department personnel and those we serve. Education/strict adherence to hand hygiene is a great starting point. Hand hygiene education should include appropriate locations to perform handwashing and materials to use. NFPA 1581 Chapters 5 and 8 provide the appropriate guidelines covering washing.5 It is also recommended, if not already provided, that wall-mounted paper towel dispensers be procured and mounted in each restroom facility. Another consideration is locating handwashing stations in the apparatus bay so you can accomplish this task before entering any of the station living quarters. An evaluation and update of your department’s infection control plan should reflect this practice per NFPA guidelines.
Another recommendation is procuring SCBA cleaning/disinfecting solutions that the SCBA manufacturer has approved and testing for efficacy. One concern regarding cleaning solutions’ efficacy is the presence on SCBA of “biofilms,” bacteria communities that exist within a protective matrix that the bacteria itself secretes. Bacteria that exist within a biofilm are often resistant to several commercially available cleaning products. Biofilm is like the plaque on your teeth; it usually requires a good scrubbing to remove it. If biofilm is present, confirm whether the manufacturer-approved disinfectant solutions can permeate the film for a desired effect. Depending on the results, add the adopted cleaning method to your department’s infection control plan.
Consider providing every member with his own SCBA face piece regulator, although we recognize this approach comes at a significant cost. NFPA 1852, Standard on Selection, Care, and Maintenance of Open-Circuit Self-Contained Breathing Apparatus (SCBA) (2019 Ed.), Chapter 6 outlines cleaning and disinfecting of the SCBA and components.6 This standard specifically mentions that “all pneumatic components shall be thoroughly dried after cleaning.”
Consider the contact time required for the disinfectant to effectively kill bacteria, followed by the rinsing and drying of the pneumatic components: The time required to clean an individual SCBA ranges between 30-45 minutes. To streamline the process, consider replacing the face piece regulator with the quick connect/disconnect component so you can place the SCBA back into service while decontaminating the contaminated regulator.
NFPA 1852, 5.1.5 does provide a list of items to be considered for any organization seeking to replace SCBA through its selection process. The first listed item to consider is “cross contamination between users and ease of cleaning/decontaminating.” Since multiple persons generally check SCBA at shift change, where exhaled breath enters the internal components, you could easily conclude that these regulators should be decontaminated daily. Further research is needed to determine and adopt an efficient and effective practice to mitigate these research findings.
This study determined that the fire service must expand its educational outreach to include SCBA cleaning and disinfecting practices as integral to infectious disease reduction. Handwashing habits are an important component in the spread of MRSA and described as “one of the most important steps you can take to avoid getting sick and spreading germs like MRSA.”7
If it hasn’t already, your organization must adopt a plan that targets methods to appropriately disinfect SCBA to reduce the spread of infectious bacteria among personnel who share this vital piece of PPE. We must incorporate these practices into an infection control plan to provide for the safety and well-being of department members. We need further research to determine the efficacy of disinfecting the SCBA and the potential to spread other organisms through shared PPE. Addressing this problem not only helps department members, it will also help those that we serve by protecting them from us as a potential infection source. We urge everyone to understand that the infection control standards we abide by not only help us but those we serve also.
So, what is bugging you? A vital piece of firefighter PPE, the SCBA, can harbor infectious disease, specifically MRSA. A suitable disinfecting technique must be researched further and incorporated into an infection control plan to further reduce exposure and the indirect spread of MRSA within and from an organization. NFPA standards will help your organization improve on your SCBA disinfecting techniques and improving your infection control plan. If these problems are left unaddressed, personnel may resort to unapproved methods to disinfect the SCBA, which could have catastrophic consequences. Our goal should be to prevent firefighters from taking infectious pathogens home to our families.
Are we are doing enough about infection control? Currently, no. Technically, there is always room for improvement; we may not be able to completely eliminate these “bugs” from contaminating our equipment, but we can reduce the amount of contamination. We still don’t have the answers to many questions following this research, but we know it clearly indicates the need to continue research to improve on current practices. We don’t want to be alarmists about infectious disease; rather we want to get the message out that an infection control program is never static and always needs improvement. Look up your organization’s infection control program; are your SCBA mentioned in it? We want to encourage you that if something is bugging you, explore it, stay curious, and begin fixing those harmful findings so our future firefighting forces continue to set the bar higher than it was set for us.
References
1. Peate, Dr. Wayne, et al. “Methicillin-Resistant S. Aureus (MRSA) in Fire Facilities: A Guide for Firefighter Management.” International Fire Service Journal of Leadership and Management, 3(2), 2009, 15–21. https://bit.ly/36J0nsW. Accessed 1 Oct. 2019.
2. Roberts, Marilyn C, and David B No. “Environment Surface Sampling in 33 Washington State Fire Stations for Methicillin-Resistant and Methicillin-Susceptible S. aureus.” American Journal of Infection Control. June 2014, 591–596. https://bit.ly/3d7NWb1. Accessed 7 Oct. 2020.
3. CDC Division of Healthcare Quality Promotion. S. Aureus in Healthcare Settings. 17 Jan. 2011, https://bit.ly/3lkxRBO.
4. Rongpharpi, S. et al. “The Prevalence of Nasal Carriage of Staphylococcus Aureus Among Healthcare Workers at a Tertiary Care Hospital in Assam with Special Reference to MRSA.” Journal of Clinical Diagnostic Research. 2013 Feb; 7(2): 257–260. https://bit.ly/3d5P13j.
5. NFPA 1581, Standard on Fire Department Infection Control Program, 2015 edition.
6. NFPA 1852, Standard on Selection, Care, and Maintenance of Open-Circuit Self-Contained Breathing Apparatus (SCBA), 2019 edition.
7. CDC. “Methicillin-resistant Staphylococcus aureus (MRSA): Cleaning and Disinfection.” (2019, February 27). https://bit.ly/34Burnl. Retrieved January 05, 2020.
Authors’ note: Thanks to the following for their assistance in this research project: Paramedic Shelly Parker for gathering swab samples; Dr. Abdul Hamood for the use of his laboratory at TTUHSC; and the participating fire department.
Shane M. Parker has been a career firefighter/paramedic in a medium-sized fire department since 2003. He has a bachelor of applied arts and sciences degree in emergency medical service management from Eastern New Mexico University and is a graduate of the National Fire Academy Managing Officer Program.
Cassie Kruczek graduated from Texas Tech University Health Sciences Center (TTUHSC) in 2013 with a Ph.D. in biomedical sciences with a concentration in microbiology and immunology. She is an assistant professor in the Department of Medical Education at TTUHSC.

