Quality of Compressed Breathing Air —How It is Maintained and Tested
features
BY
“Safety Equipment May Not Be So Safe,” ran a national headline last July when a government study disclosed that at least 10 percent of all self-contained breathing apparatus was in some way defective.
As the fire service encounters mask problems and searches for solutions, what may be overlooked is this basic concern: the quality of the breathing air itself. How is that quality defined? How is it maintained? How can it be tested?
There is a single national criterion for breathing air purity. The Compressed Gas Association (CGA) has issued “Commodity Specification for Air” No. G-7.1, 1973 edition, which lists nine different grades of breathing air in gaseous form. This was adopted in 1973 by the American National Standards Institute as ANSI Standard Z86.1. By reference, OSHA General Safety Standards (1975 and 1977) and the NFPA have adopted this same specification, Grade D, as the minimum quality level for compressed breathing air.
Grade D limits carbon monoxide (CO) content to 20 parts per million (ppm); carbon dioxide (CO2) to 1000 ppm; oxygen to between 19.5 and 23.5 per cent; no more than “slight” odor; condensed hydrocarbons not more than 5 milligrams per cubic meter of air. No limits are stated for other possible impurities. California State standards are somewhat more restrictive, limiting CO2 to 300 ppm and CO to 10; odor must be nondetectable. To maintain these quality levels requires a breathing air purification system, or BAPS, between the compressor and the bottle filling station.
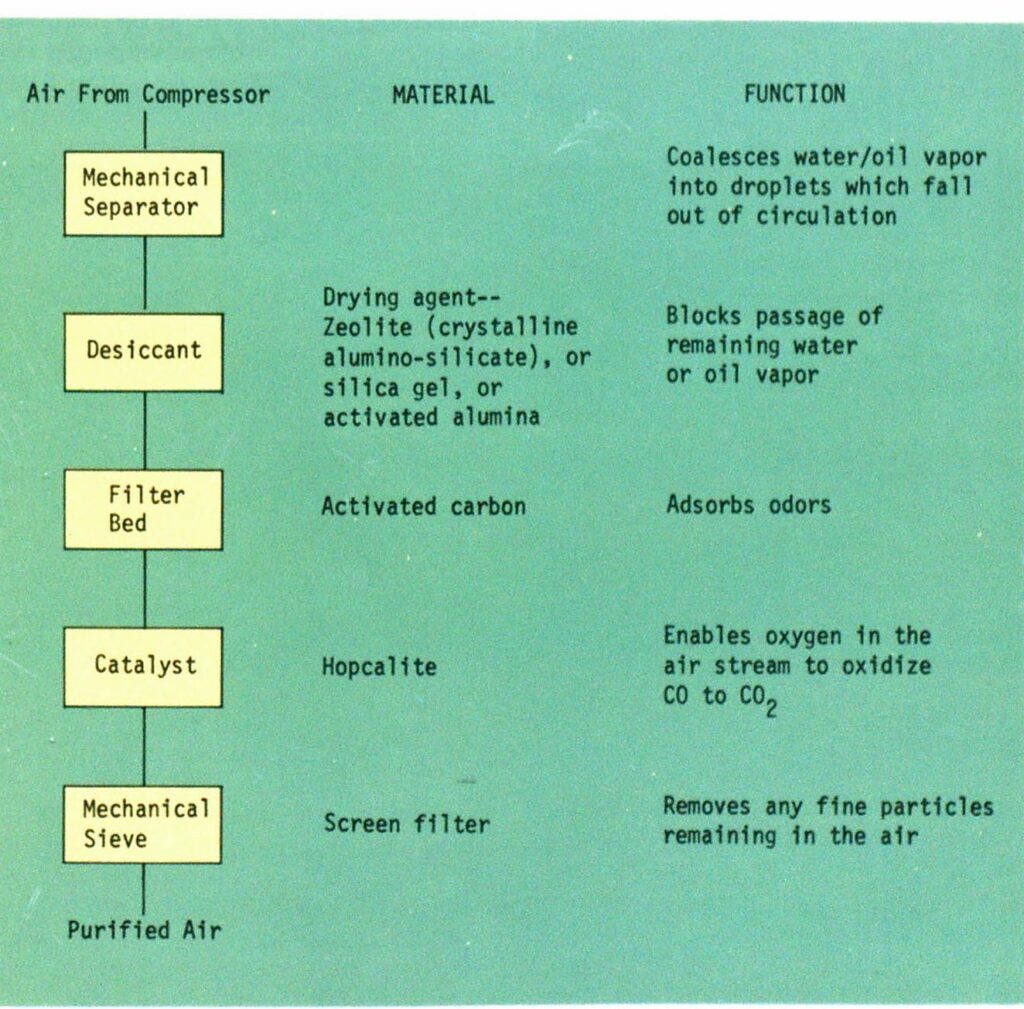
More than a filter
A BAPS is much more than a simple filter. In the average metropolitan area, it’s been estimated that each cubic foot of air holds more than 4 million dust particles, 80 percent of them less than 1 micron in size (a micron is a thousandth of a millimeter). Besides the other contaminants mentioned earlier, there are also hydrocarbon gases, ozone, nitrogen oxides (NOX), and sulfur compounds. In addition to these, the compressor itself may produce oil vapor, carbon, and metallic wear particles. Mechanical filters will block very little of this.
Figure 1 shows what a commercial BAPS generally includes. Similar components were developed by NASA’s crew systems division for air systems in the 4000-5000 psi range (see Fire Engineering, August 1974, page 46).
One of the more important elements in this BAPS is the catalyst that converts toxic CO to relatively harmless CO2. The commonest version is known as Hopcalite. This material was developed in August 1918 through research growing out of inadequacies in World War I gas masks—and their use in underground mine rescue work—by a team of scientists from Johns Hopkins University and the University of California (hence the “Hop” and “cal” portions of the name). It is a mixture of 50 percent manganese dioxide with cuprous oxide, silver oxide, and cobaltic oxide, in loose granular form. Conversion of CO takes place at the surface of these granules. That is why Hopcalite works properly only if kept dry. If condensed water vapor coats the granules so that the air to be purified cannot reach their surface, the reaction cannot take place. The catalyst is said to be “poisoned.”


Ironically, Hopcalite was the catalyst in the all-service, or canister, mask that was widely used until “outlawed” in 1971. Failures of those masks were caused not by any defect in the catalyst, but by its being subjected to conditions it was never designed to deal with.
CO concentration effect
When Hopcalite was first developed, air containing as much as 1 percent CO was considered badly contaminated. Hopcalite will remove 90 percent of the CO from air containing only 1/4 percent of the gas. But when this fraction rises to more than 12 percent, as is known today to occur at many fires, the catalyst is no longer effective. That’s why it is considered inadequate for present-day mask service.
When CO content is quite low, however, as it should be in any breathing air supply system, Hopcalite performs quite well. As Figure 1 shows, it is always preceded in the air flow by a drying agent to remove the poisoning water vapor.
All the purification agents in a BAPS are usually sealed within a single replaceable cartridge, often equipped with a visual indicator to show when the elements are becoming exhausted. It is then time to replace the cartridge. But these signals should not be relied on as the sole indicator of system condition.
Even though the manufacturer may suggest a cartridge replacement interval, the system operator will have to work out his own schedule for his own conditions. Suppliers recommend keeping an accurate log of the number of bottles filled (or of compressor operating hours) to determine actual cartridge capacity.
Records to be kept
System operators should also keep records of:
- Air quality test results.
- Purification cartridge changes.
- Compressor servicing.
- Any other problems observed, such as the appearance of occasional odors in the air, bottle defects, etc.
Even though still internally usable, some types of purifier cartridges are subject to chemical deterioration of the casing, requiring renewal before any air quality indicators or monitors might call for it.
The amount of air that can be safely processed through any cartridge varies with both temperature and pressure (see Figure 2). As this illustration shows, cartridge life is greatly extended if the entering air is cool. Getting the air down to 65° to 85° F normally requires a high pressure aftercooler (either air or water cooled) in the system. If the air entering the purifier is much above 100°F, some of the purifying elements may not be effective.
Concern about air source
Proper replacement interval also depends on the purity of the air source. As in the old all-service mask, purification chemicals are not capable of removing impurities from air in unlimited quantities. They are not intended, as one manufacturer puts it, “to be a substitute for good safety practices.” Particular care must be taken in using mobile air supply systems at fires or other emergencies where the air being drawn into the compressor may be unusually contaminated by smoke or toxic gases. Vehicles with engines running should be kept at a distance to avoid overloading intake air with carbon monoxide.
Using an air source which is as pure as possible to begin with becomes extremely important when there is no BAPS at all. For example, in one Eastern state, it waS once common practice for a number of volunteer fire departments to have their bottles filled by local welding supply houses under uncertain conditions. It was sometimes done in equipment garages filled with exhaust fumes. More recently, one fire department in that area has installed a purification system and now recharges bottles for neighboring departments.

Photo by Eagle Air Systems

Photo by American Bristol, Inc.

Photo by Mako Compressors, Inc.
Lacking such a system and dependent on commercial compressed gas suppliers, a fire department needs to take periodic tests to ensure that the air meets purity standards. Speaking before the Metropolitan Committee of the IAFC in May 1979, Pat McLaughlan of NASA cited the lack of routine sampling of air purity as one of the serious respiratory protection problems for fire fighters. He called for emphasis on purification system performance— which cannot be checked unless the air supplied by the system is tested.
Checking air quality
It may be possible, especially in metropolitan areas, to send an entire bottle to a municipal or state testing agency which can certify the results. The ANSI/CGA specification details the methods to be used for testing an air sample for each of the common contaminants.
In Milwaukee, for example, the fire department shop fills 8000 bottles a year.
When the present BAPS was first installed in 1972, according to Ross Lanza, assistant shop superintendent, “We checked air purity every 1000 bottles. It has always tested OK. Now, we find it’s necessary to change the filter only twice a year.”
At each test, a filled bottle is sent to the state for testing. Certain checks can be made by built-in monitoring equipment between tests.
Says one leading manufacturer of breathing air supply equipment, “We recommend that each system have a monitor for moisture and carbon monoxide. These can be either electronic or chemical/mechanical. By using these monitors along with an hour recording meter, the operator has sufficient notice to change the purifier cartridges and elements before the system can get saturated.”
The available features of CO monitors include:
- Operation on either 110-vac or 12-vdc power for fixed or mobile systems.
- Visual and/or audible alarms, local or remote.
- Adjustable alarm setting, usually in the 0-50 ppm of CO range.
- Meter to display CO concentration.
- Regulator for coupling to either high or low pressure air systems.
- Electrical interlocking to shut down the compressor if CO concentration gets too high.

Photo by Robert B Milnes
Similarly functioning monitors are available to check the moisture content of the air.
OSHA Title 29, Part 1910.134, requires that oil-lubricated compressors either have a carbon monoxide alarm or that the air output be “frequently” tested (one interpretation being whenever the compressor is started) for CO. The reason is that oxidation of oil vapor in the air is a common source of CO in the compressor output.
Oil vapor itself, though of limited toxicity, can cause discomfort and even nausea. There are reports of lipoid pneumonia having resulted from breathing oil vapor. Partial oxidation of lubricating oil and its additives causes a characteristic unpleasant odor in the air. The State of Louisiana has forbidden use of oil-lubricated compressors for breathing air. Nonlubricated or water-lubricated units must be used.
Importance of dry air
As for water vapor, it is true, as the International Society of Fire Service Instructors has pointed out, the ANSI/ CGA standard sets no specific limits for water content or dew point of Grade D air. The dew point determines the required capability of the drying agent used in the purification system.
However, BAPS suppliers realize the importance of dry air simply because the operation of their equipment depends upon it. In a typical system, the dew point is about minus 100° F before the air enters the catalyst bed. Manufacturers recommend this point not be allowed to rise above minus 40 for surface use. For diving, it should not be above minus 60.
To illustrate the air dryness achieved by such a limit, air with a dew point of minus 100°F contains only 1/10 pint of water per million cubic feet. So if a proper catalyst bed and upstream dessicants are used in the BAPS, moisture content of the output should be no problem despite what may appear to be a loophole in the standard.
Another bad effect of water vapor is rust inside air bottles. This will not take place in the newer fiberglass/plastic versions, but rust can still be carried over from cascade system cylinders. This may be difficult to clean out. It can clog mask valves and regulators. Moreover, moisture can lead to dangerous loss of bottle strength through stress/corrosion cracking.
Moisture triggers warning
Actually, the usual color change indicator warning of the need for cartridge replacement is not a gas sampler but a moisture indicator. In a typical system, this operates when a dew point of minus 30°F is reached. This warns the operator that the desiccant is saturated and no longer able to remove enough moisture from the air for effective operation of the catalyst downstream.
If other testing capability is unavailable, a fire department can do its own testing with the sampler pump/detector tube equipment or “stain tube kit” offered by breathing air equipment dealers. This kit includes a small handoperated plunger pump with a piston travel that can be pre-set to any one of several standard volumes of air to be sampled. A glass detector, tube is inserted into the end of the pump. This tube, about the size of a pencil, contains a chemical which undergoes color change in the presence of the impurity being checked. How far this color change spreads along the length of the tube depends on the amount of impurity in the air sample. Tubes are made for CO, hydrogen sulfide and several other contaminants.
For carbon monoxide, these items typically cost $80 to $90 for the pump itsself with some accessories, plus a little over $1 each for detector tubes. Shelf life of the tubes is about 2 1/2 years.
Test methods
The user can bleed air to be tested into a plastic bag. The tube is then put into the bag and its glass tip broken off to admit air when the pump is operated. Another method sometimes used is to attach a tee fitting to the charging whip or to a full air bottle. Two small lines of plastic tubing, just big enough to fit over the detector tube, extend from this fitting. The air supply valve on the whip or bottle is then cracked open. Air is thus made available to the detector tube through one line while excess flow vents through the other line.
Although these kits will test for most contaminant gases, there is no such simple means for checking oil vapor in the air. This must be done in a lab by an industrial hygienist.
Before preparing a test sample for shipment to a lab, make sure the container itself is uncontaminated. Containers should be carefully chosen, cleaned, and dried before use. When a cascade system is used, extra sampling may be needed to make sure that possible contamination of the cascade cylinders is distinguished from that in the air supply itself.
When air bottles are needed at the scene of an emergency, there is no time to check air purity. The only way to be sure of breathing air quality is to make sure the supply system includes an adequate BAPS and to make periodic checks to see that it remains adequate.

