By Kenneth W. Fent, Gavin P. Horn, Katherine M. Kirk, and Michael B. Logan
Firefighter personal protective equipment (PPE) provides excellent protection from environmental hazards during firefighting, but the protective ensembles often become contaminated with combustion by-products while serving this purpose. Few scientific research studies have attempted to quantify this contamination, which may consist of nonvolatile, semi-volatile, and volatile compounds. At normal environmental conditions, nonvolatile compounds exist primarily as solids; semi-volatile compounds are found as both condensed (liquid or solid) and gas phase; and volatile compounds readily generate vapors that can be inhaled because of their high vapor pressure. As examples, flame retardants are generally nonvolatile, whereas low molecular weight polycyclic aromatic hydrocarbons (PAHs) are semi-volatile and single-ring aromatic hydrocarbons (like benzene and toluene) are volatile organic compounds (VOCs).
 |
| (1) Enclosure used by NIOSH to sample off-gassing PPE ensembles. After closing the lid, the metal canister collected VOCs for analysis. (Photo courtesy of the National Institute for Occupational Safety and Health.) |
During firefighting, nonvolatile contaminants present an exposure hazard mainly through firefighters coming in physical contact with the substances and subsequent dermal absorption and hand-to-mouth ingestion. Volatile and semi-volatile contaminants may also present an exposure hazard through the inhalation route, whereby the PPE acts as a temporary adsorptive material for airborne contaminants (gases and particles) that may then be released into the air during postfire activities. These evaporating contaminants may be inhaled after a firefighter doffs his self-contained breathing apparatus (SCBA).
This potential inhalation route of exposure has not been previously investigated. Hence, investigators from the National Institute for Occupational Safety and Health (NIOSH) in the United States and Queensland Fire and Emergency Services (QFES) in Australia separately set out to study the accumulation and off-gassing of combustion by-products on firefighters’ PPE ensembles. Results from these two independent studies were recently published in the same issue of the peer-reviewed Journal of Occupational and Environmental Hygiene (June 2015).
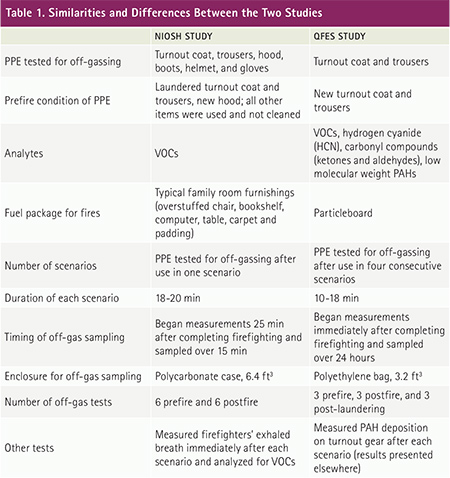
Table 1 describes the similarities and differences of these two studies. The basic premise of both studies was the same-to test PPE ensembles for off-gassing contaminants before and after being worn while fighting fires in structures with typical room-and-contents furnishings (NIOSH) or common training fuels (QFES). QFES investigators also tested PPE ensembles after laundering per manufacturer’s recommendations to determine whether off-gas concentrations returned to normal background levels.
Results
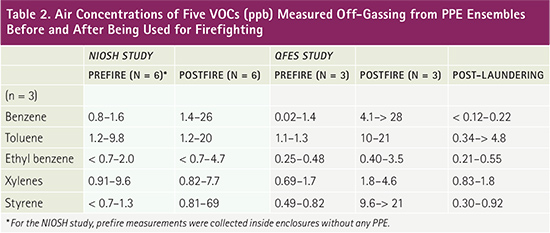
Investigators in both studies measured elevated levels of a variety of VOCs off-gassing from PPE ensembles postfirefighting (compared to prefirefighting levels), with similar findings for benzene, toluene, ethyl benzene, xylenes, and styrene (Table 2). QFES investigators also measured elevated levels of methyl isobutyl ketone, acetaldehyde, crotonaldehyde, benzaldehyde, and hydrogen cyanide (HCN). HCN was measured at concentrations up to 10× higher than any other compound. NIOSH investigators found a relationship between off-gas concentrations and exhaled breath concentrations of benzene, toluene, ethyl benzene, xylenes, and styrene. QFES investigators found that most off-gas concentrations returned to normal background levels after laundering.
 |
| (2-3) The enclosure used by QFES to sample off-gassing structural firefighting ensembles. The ensembles shown are post-laundering. After sealing the enclosure, sampling pumps were operated through the plastic enclosure (photo 3) to commence sampling. (Photos courtesy of Queensland Fire and Emergency Services.) |
Discussion
Inhalation exposures are likely to occur when firefighters are not wearing SCBA or alternative forms of respiratory protection and they do the following:
- Continue to wear a turnout coat and trousers when packing up and loading the apparatus.
- Rehab near used PPE ensembles or, if PPE is not fully doffed, in the rehab area.
- Change air cylinders between work cycles on the fireground.
- Wear or store PPE ensembles in the cab of the apparatus or personal vehicle.
- Spend time in a location at the firehouse where unlaundered PPE ensembles are stored.
Off-gas exposures are likely to be highest immediately following use of PPE in a fire, when firefighters are in close proximity to PPE, and especially if firefighters and their PPE are in an enclosed space.
The findings from these studies are important because they demonstrate that off-gassing from contaminated PPE can extend exposure time beyond the fireground and, therefore, represents another source of exposure for firefighters that should be managed. Off-gassing air concentrations in both studies were below applicable occupational exposure limits, although higher concentrations may have been measured if testing occurred immediately after firefighting and culminated within 15 minutes thereafter.
Potential exposures from off-gassing PPE, albeit typically brief, consist of multiple compounds, including known irritants (e.g., aldehydes), chemical asphyxiants (e.g., HCN), and carcinogens (e.g., benzene). The potential additive or synergistic effects of these multiple exposures are largely unknown. Irritants can cause inflammation in the lungs, and chemical asphyxiants reduce the oxygen-carrying capacity of the blood. In addition to the effects on the respiratory system, both irritants and chemical asphyxiants can place added stress on the cardiovascular system.
Firefighters appear to have a higher risk of various types of cancer and, therefore, should minimize their exposures to potential carcinogens as much as possible. Appropriate storage and timely laundering of PPE could substantially reduce cumulative exposures to these compounds over the duration of a firefighting career.
The significant relationship between off-gas concentrations and exhaled breath concentrations found by NIOSH suggests that contaminated PPE contributes to firefighters’ absorbed dose even during the short time (few minutes) firefighters doff gear. However, the exhaled breath concentrations measured by NIOSH could also be partially attributed to direct uptake of VOCs through the skin during firefighting. Further research is required to determine the relative effects contributed by each of these exposure pathways.
Both studies focused primarily on measuring volatile substances. Semi-volatile and nonvolatile compounds could also contaminate firefighters’ PPE ensembles and potentially transfer to the skin of firefighters where they could be absorbed dermally or ingested. Unlike VOCs, which will mostly evaporate from surfaces within a few hours, semi-volatile compounds could evaporate over much longer periods of time and pose a longer duration inhalation hazard. Furthermore, PAHs and other carbonaceous substances that contaminate PPE may act like activated carbon, adsorbing VOCs in the fire atmosphere and then slowly releasing them over time.
Research aimed at a greater understanding of where and why the highest firefighter exposures occur during fire response activities may assist in lowering PPE contamination levels. Further research is needed to fully characterize the magnitude and composition of contamination on firefighters’ PPE ensembles, with an emphasis on highly persistent compounds like flame retardants, dioxins, and phthalates. An assessment of the effectiveness of decontamination and laundering at removing such contamination is also needed.
The following resources contain additional information on this topic:
- Kirk KM and Logan MB. (2015) “Structural Fire Fighting Ensembles: Accumulation and Off-gassing of Combustion Products,” J Occup Environ Hyg, 12:6, 376-383.
- Fent KW, Evans DE, Booher D, Pleil JD, Stiegel MA, Horn GP, and Dalton J. (2015) “Volatile Organic Compounds Off-gassing from Firefighters’ Personal Protective Equipment Ensembles after Use,” J Occup Environ Hyg, 12:6, 404-414.
- NIOSH (2013) “Health Hazard Evaluation Report: Assessment of Dermal Exposure to Polycyclic Aromatic Hydrocarbons in Fire Fighters.” http://www.cdc.gov/niosh/hhe/reports/pdfs/2010-0156-3196.pdf.
The two independent studies by NIOSH and QFES clearly demonstrate that a variety of volatile substances can accumulate and subsequently off-gas from firefighters’ PPE ensembles. The off-gassing compounds could result in inhalation exposure for firefighters if they remove SCBA and remain in close proximity to their PPE postfirefighting, particularly if this occurs within an enclosed space. Firefighters and fireground support personnel should take measures to minimize these exposures. These measures include rehabbing away from their used ensembles; storing their ensembles in dedicated, well-ventilated areas; and, if possible, transporting ensembles outside the apparatus cab or personal vehicle after responding to a structure fire. Prompt laundering of ensembles after use in structural firefighting operations is an additional effective measure in reducing postfirefighting exposures to volatile products of combustion.
KENNETH W. FENT, PhD, CIH, is a research officer at the National Institute for Occupational Safety and Health and a lieutenant commander in the U.S. Public Health Service. He has performed numerous evaluations of firefighters’ chemical exposures.
GAVIN P. HORN, PhD, is the director of IFSI Research at the Illinois Fire Service Institute-University of Illinois at Urbana-Champaign and a volunteer firefighter/engineer with the Savoy (IL) Fire Department. He has performed many studies focused on firefighter health and safety.
KATHERINE M. KIRK, PhD, was a scientific officer with Queensland Fire and Emergency Services for 10 years, providing operational support for hazardous materials emergencies. She works at QIMR Berghofer Medical Research Institute and is a volunteer firefighter. Her research interests include analysis of emergency responder exposures to by-products of fire, firefighter respiratory protection, and hazard prediction modelling.
MICHAEL B. LOGAN, PhD, is the director of the Queensland Fire and Emergency Services (QFES) Research and Scientific Branch. The Research and Scientific Branch responds to about 450 incidents each year and undertakes a research program investigating firefighter exposures and other areas of interest to emergency preparedness and response.
IFSI Study Underway on Firefighter Health
Firefighter Health and Safety: Report: Firefighters’ Hoods Seen as Cancer Risk
Reducing the Incidence of Cancer Among Firefighters: Jacksonville’s Best Practices
Firefighter Health and Safety: Report Focuses on Firefighter Gear , Cancer

