
By Jeffrey O. Stull
Between fighting fires and responding to other emergencies, firefighters expose their personal protective equipment (PPE) to a wide range of toxic chemicals, biological pathogens, and other hazardous substances. It is well recognized that these contaminant exposures can pose significant dangers to firefighters’ immediate and long-term health, with an increased risk of a cancer diagnosis topping the list of dangers. The contaminants in soiled PPE further cross-contaminate everything with which they come in contact.
As a consequence, turnout clothing is being cleaned more frequently; however, we still don’t know if current or new cleaning procedures adequately remove such contaminants from PPE. Although general cleaning procedures have been established in National Fire Protection Association (NFPA) 1851, Standard on Selection, Care, and Maintenance of Protective Ensembles for Structural Fire Fighting and Proximity Fire Fighting, more science is needed to support, clarify, and enhance those procedures.
In late 2015, a four-phase PPE research project was initiated through a Department of Homeland Security Assistance to Firefighters Grant (AFG) research program by the Fire Protection Research Foundation (FPRF) to identify and establish procedures that ensure optimum contaminant removal from firefighter PPE. The principal output of the research has been to develop specific test methodology that can be used to assess the removal of specific priority contaminants from clothing in wash processes. The research team has created a “kit” that will be used at a cleaning facility to assess specific cleaning procedures. The kit contains specially prepared clothing material swatches infected with known levels of different contaminants. The swatches are placed in sample clothing and are subjected to cleaning at an individual cleaning facility. The kit is sent to a qualified laboratory, where the swatches are removed from the sample clothing and analyzed for remaining concentrations of carcinogenic low-volatility organic chemicals (like polynuclear aromatic hydrocarbons and certain plasticizers), toxic heavy metals, and representative infectious bacteria. This information is then used to quantify the decontamination effectiveness of the respective cleaning process.
Because the kit approach can be applied anywhere, it is being positioned as a way of qualifying how well independent service providers (ISPs), manufacturers, and fire service organizations clean turnout clothing. The procedures also provide direction for selecting appropriate machines, detergents, and processes as provided by industry and will aid in advancing firefighter protective clothing cleaning technology. Information gained by the study is further being used to examine current practices for setting the wash temperature, number of rinse cycles, and utility of clothing pretreatments and process enhancements such as using ozone as a sanitizer. Ultimately, the goal of the research is to reduce firefighter exposure to contaminants. The results of the study will be used to recommend changes to NFPA 1851 that result in effective cleaning and removal of contaminants, which will, in turn, lessen the exposure of firefighters to contaminants held in clothing and minimize transfer to their equipment, vehicles, and living areas.
Along the way, through the efforts undertaken in this investigation, by embracing the research of others, and by learning from manufacturers and end users, the substantial amount of information that has been gained will help improve the awareness and acceptance of new best practices within the fire service—all supporting the end goal of reducing every firefighter’s exposure to contamination. Although we do not have all of the answers, there are a growing number of approaches that departments can implement to improve contamination control as part of their PPE programs. These approaches, what we call “Key Takeaways,” are a combination of new ways of thinking and evolving procedures that can be adapted to each organization.
Key Takeaways
#1: Recognize Fires as Hazardous Materials Incidents
We like to say that structural fires are more “hazmat” than hazmat incidents. This reflects a reality of today’s fire exposures. Fires have always been dangerous, but it has only been in the past few decades where firefighting has been associated with chemical exposure and where the focus has shifted from simply burn protection to concerns about contamination. NFPA statistics show that there were approximately 442,000 hazmat incidents in 2015; comparatively, there were more than 1,345,500 structural or other types of fires that departments respond to annually. Nevertheless, despite this large difference in the number of incidents, the key distinction is that responding to a fire almost always involves operating in an immediately dangerous to life or health (IDLH) environment requiring the use of self-contained breathing apparatus (SCBA) and almost certainly having some level of skin exposure to the hazardous chemicals contained in smoke from other particulates and from liquids encountered on the fireground.
In contrast, the large majority of hazmat incidents entail detailed assessments of potential respiratory or dermal exposures; procedural response approaches at the emergency scene; and the use of PPE that pretty much limits any contact with the chemicals, which are also generally few in number and present at extremely low concentrations. Although many experienced fire service members will contend that these are two completely different types of operations, structural fires impose more frequent and generally higher risk events in terms of their chemical exposures than most hazmat incidents.
Unfortunately, unlike hazmat PPE, most structural firefighting PPE will not fully protect the wearer from hazardous substance exposures. This was most aptly shown by the research performed for the International Association of Fire Fighters (IAFF) at Research Triangle Institute where a testing subject wearing a used firefighting ensemble and exercising inside a closed test chamber with aerosolized and fluorescently tagged particles showed dramatic deposits of the surrogate smoke particles on the subject skin and under clothing after the 30-minute exposure. This testing only demonstrated the obvious: that firefighters are routinely exposed to small soot particles. They are also exposed to fire gases that consist of the combustion products evolving from the fire and water and other liquids that become contaminated at the scene.
Many studies document the various types of contaminants on the fireground, which are not limited to the active phases of structural fires. During overhaul, some of the highest levels of contaminants can still be present, resulting in significant respiratory and dermal exposures. Even smaller-scale fires, such as those involving vehicles and dumpsters, can still present an array of hazardous substances. Modern materials and the array of contents in structures and other products or areas that catch on fire pre-sent an increasingly complex mixture of combustion products, with most of these substances harmful for human exposure, including a number of known carcinogens. There is an exceedingly long list of substances that include a variety of hazardous compounds such as heavy metals, inorganic acids/bases/salts, volatile organic chemicals (benzene, formaldehyde, and hydrogen cyanide), and semivolatile organic compounds (acenaphthene, dioctyl phthalate, and pentachlorophenol), which are further broken down into categories such as polynuclear aromatic hydrocarbons (PAHs), phthalate-based plasticizers, dioxins, halogenated flame retardants, and particulates (asbestos and lead dust). At any given fire, many of these substances are likely to be present in various concentrations and potentially come in contact with firefighters and their PPE.
Accepting fires as hazmat incidents is a good first step in the process of controlling exposure to contamination. It creates a change in the way of thinking on the fireground by recognizing that contamination is present and seriously hazardous. It further leads to specific steps to apply for its control.
Structure fires and other fire events create a large number of hazardous chemicals, many of which remain as persistent contamination in turnout clothing.
In a hazmat incident, the following important approaches are applied:
Establish control zones at the emergency scene. Using the language of hazmat, “Hot,” “Warm” (or contamination reduction), and “Cold” zones are established where specific PPE usage within the hot and warm zones is strictly enforced and unprotected personnel are kept away from exposure hazards; contamination is not allowed to go from the hot to the cold zone.
Use the appropriate PPE, and wear it correctly to minimize any exposure for all activity in the hot and warm zones. This means wearing the SCBA wherever there are potential inhalation exposure hazards and ensuring that the helmet ear flaps are down, the coat collar is up, the hood properly overlaps the SCBA face piece, and all closures are properly fastened.
Reduce exterior contamination and isolate PPE before you leave the fireground. This involves applying procedures for preliminary exposure reduction of PPE contamination before leaving the warm zone.
Pay careful attention to the containment and transport of PPE. Place PPE in a bag and container after doffing until it can be fully decontaminated or assessed for retirement and disposal.
Apply procedures for ensuring proper decontamination of PPE. Properly clean PPE with techniques that demonstrate removal of contaminants. Any reuse of PPE should not result in additional firefighter exposure.
Do not reuse PPE that cannot be cleaned properly. In cases where decontamination is not possible or the types of contaminants pose high risks, condemn and dispose of PPE.
These steps represent a significant transformation of fire service protocols for many departments. It is not an easy transition, yet several organizations have already begun to apply standard practices toward achieving these approaches. A key missing element is being able to measure and determine “How clean is clean?” and that begins with understanding how PPE is contaminated and how that contamination spreads.
#2: Understand PPE Contamination and Exposure
Every emergency response represents a possible contamination event. Firefighters operate in all types of environments, whether it is an emergency medical services (EMS) call, a dumpster fire, or a structure fire. If there is exposure to gases or vapors, liquids, or particles, these substances will get onto clothing and in many cases remain on the clothing until it is adequately cleaned. In some cases, some substances may not come off the clothing even during cleaning.
Gases and vapors will expose all surfaces of the PPE and generally will easily penetrate any textile component. Coated or laminated materials such as trim or moisture barriers together with hard surface items such as helmet shells will physically retard gases and vapors, but many of the substances can still permeate materials on a molecular basis. This is also true for leather and rubber materials. While leather is porous like fabric, many chemicals are soluble in rubber.
Liquids will enter any gap in the material or clothing, particularly through interface areas. For textile fabrics, once the outer surface is wetted, the liquid will penetrate. Moisture barriers, coated materials, and rubber prevent liquid penetration, but many nonwater liquids will penetrate gaps more easily than water because they have lower surface tensions. Liquid that soaks a material will spread to other areas of the clothing by wicking, thus spreading contamination beyond the point of clothing entry.
Particulates can range from asbestos fibers to dry wall dust, but the largest contributors of particulate contamination at the fire scene are the carbon particles from incomplete combustion. These carbon or soot particles adsorb fire gases and hold them in small pores within the particles, making them more dangerous than just the plain carbon. While many particles are of a size that is visible, many are submicron size and easily get into any porous surface or gaps in the clothing ensemble where there is no barrier. The darkening of light clothing fabrics is evidence that soot has accumulated inside the material, not just on the surface. As shown in the IAFF fluorescent particle testing, these particles can travel relatively long distances inside the clothing ensemble, even depositing in the groin area after entering through the pant-to-boot interface. The particles move inside the clothing when it is worn because the wearer moves, and that movement creates air currents that transport the particles anywhere there are air gaps.
Longer exposures produce higher levels of contamination. Yet, the extent of contamination is also heavily dependent on the nature of the substances involved. For example, oily, tarry substances created by high heat will tend to bond to clothing materials more readily, especially as the clothing item cools.
Soiled clothing picks up more contamination. Clothing that is clean may offer more surfaces for contamination, but many forms of contamination on clothing offer compromised materials that can become more soiled or can readily pick up other forms of contamination. One way to think of soot is as a chemical “magnet.” This is most often seen when soot in fabrics continues to pick up gases and vapors from the fire environment. In essence, it is easy for dirty clothing to be more soiled than clean clothing. In addition, the soils on clothing often negate whatever repellent properties a clothing fabric might have. The finishes on clothing fabrics and some other components can also wear down over time, making soiling more likely to occur.
All contamination seeks to be diluted. This is a principle called chemical mass transfer. The best example is if you took an open container of a volatile chemical (like acetone) and placed it inside a closed room. Over time, the chemical would evaporate and eventually would be equally distributed at the same concentration throughout the room. So, whereas the chemical is initially in a very concentrated state in the container, it becomes significantly less concentrated and spreads over a broader area over time. This principle also applies to contamination. PPE becomes contaminated on the fireground, picking up fire gases, absorbing liquids, and accumulating particles. However, as firefighters leave the fireground, some of the substances begin to dilute when presented with fresh air.
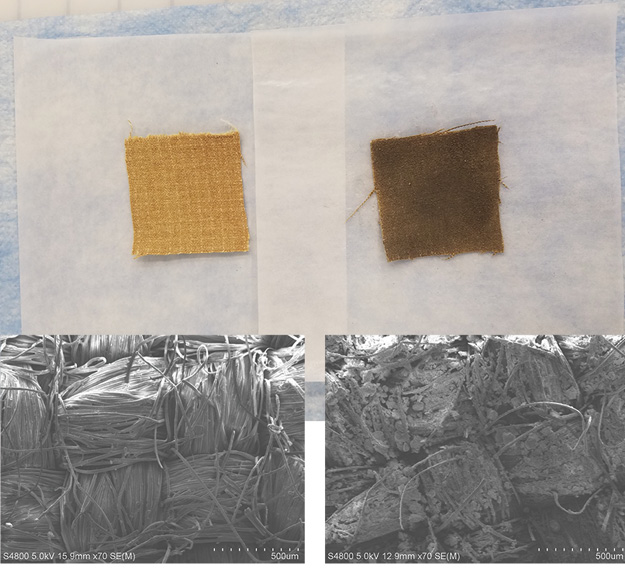
(1) Clean and soiled outer shell fabric swatches side by side with electron microscope photographs showing dirt particles embedded in the soiled swatch under extreme magnification. (Photo courtesy of the NIOSH National Personal Protective Technology Laboratory and Health Effects Laboratory Division.)
One of the dangers of contaminated PPE is that the PPE materials begin to off gas after the response. This off gassing is worse immediately after exiting the contaminated environment and the reason it is important to remain on air until steps have been taken to mitigate some of the gross exposure from the contamination on the PPE. Any chemical that is volatile will continue to evaporate and enter your unprotected respiratory tract, resulting in an acute exposure. Given the amounts of chemicals that are bound to the clothing following the fire, this can result in extended exposures until you remove the clothing.
Other forms of the contamination remain tightly bound to the clothing. This occurs as the result of chemicals that have directly permeated or penetrated the materials or were absorbed onto soot particles that remain in and on the PPE materials. Chemicals that do not easily evaporate, generally referred to as semivolatile chemicals, are persistent and create longer lasting forms of contamination. These forms of contamination will also dilute but at a much slower rate than volatile chemicals. Their continued presence in or on the PPE items results in a more chronic exposure, either through inhalation or dermal exposure. Many fireground substances are toxic through skin absorption, which can be accelerated by the increased temperature and sweating common with the use of firefighting protective ensembles. Furthermore, chronic exposures are judged problematic because, for some substances, long-term exposures at low levels can result in accumulation of those substances in the body.
Persistency is also a characteristic of several forms of biological contamination (like HIV and MRSA). Firefighters who engage in emergency medical operations, usually in the form of transporting victims from an accident scene or extrication, realize the hazards of bloodborne pathogens and the need to apply Universal Precautions, where all blood and body fluids are assumed to be potentially infectious. However, it is important to further acknowledge that several other encountered microorganisms, all of which are invisible, can remain viable on clothing long after the exposure and can potentially result in infection of the firefighter through continued contact. Examples are MRSA from victim contact, E. Coli from use of PPE in flood water, and Ebola from a first responder transport of a suspected Ebola virus disease victim. Many microorganisms can remain alive and infectious for up to several weeks following contamination.
Your PPE is your work clothing, and you are working in a hazardous environment that provides many opportunities for multiple types of contamination. This contamination poses real dangers for both acute and chronic health conditions. Appropriate cleaning and decontamination of the PPE, combined with proper hygiene, are absolutely necessary to reduce your risks of exposure.
#3: Begin Contamination Control on the Fireground
After your fireground activities conclude, it is essential to take steps to reduce your exposure to fire or another emergency contamination as much as possible. Wearing your contaminated gear in the apparatus cab back to your station or in a personal vehicle to your home results in spreading that contamination to other surfaces and creates new ways for continued exposure to the same contamination without even having to wear the clothing. Worse, it now can affect fellow firefighters, the community, and your family through secondhand exposures to the same contamination.
The preceding section should convince you that it is not a reasonable expectation that the wearing of PPE is sufficient to limit your exposure to most hazardous substances you will encounter on the fireground. Certainly, your SCBA, when worn, provides respiratory protection; but SCBAs are not always worn during the entire time you are exposed to contamination, particularly during overhaul. Moreover, a significant number of chemicals absorb through skin and cause both acute and latent toxic effects. While it is true that gear has evolved extensively over the past several decades, its ability to prevent skin exposure to many fireground contaminants is quite limited. Furthermore, the contamination of exterior surfaces and inner layers of firefighter protective clothing and equipment results in exposure well after the incident if that contamination is not removed.
Structural firefighting protective clothing does include a moisture barrier layer throughout most of the ensemble elements—garments (coat and pants), gloves, and footwear are all required to have these materials to prevent liquid penetration through these ensemble items. Moisture barriers attenuate many contaminants but do not protect against all chemicals. Certainly, the helmet shell is relatively impervious itself for protecting much of the firefighter’s head; the SCBA face piece also protects the majority of the firefighter’s face. Still, several parts of the ensemble provide penetration pathways for smoke particulates and vapors to reach firefighters’ skin. Helmet ear covers, coat and glove wristlets, and all but some of the new hoods lack any form of barrier material. In addition, while garment closures are designed to be resistant to liquid penetration, these features are not airtight. Moreover, interface areas between gloves and coat sleeves, footwear and pant trouser ends, the coat and pants, and the face/head/neck interface area are all relatively open to airborne fireground contaminants.
Based on this information, for operations in proximity to a fire or external contact with other sources of contamination, you must assume that exposure has occurred and undertake specific steps to lessen the effects from the exposure. To control contamination on the fireground, the following steps are recommended:
Remain on air until it is safe to remove PPE. It is safe to remove PPE when techniques have been applied to remove exterior contamination. Certainly, the inclination is to cool down as much as possible by removing your face piece and opening your clothing, but these actions increase yours risks of inhaling chemicals that are off gassing immediately following your exit from the fireground.
Apply techniques to remove some exterior contamination. Although not full cleaning or decontamination, brushing the debris from the exterior of your gear will lessen the contamination. Better yet, as shown by preliminary information released in a recently completed study performed by the National Institute for Occupational Safety and Health (NIOSH), Illinois Fire Service Institute, and Underwriters Laboratories, spraying the exterior of the ensemble with a soap/water solution followed by rinsing has been shown as relatively effective in removing surface contamination. This type of “gross decontamination” is not complete cleaning, but it will lessen the overall amount of contamination in the clothing by removing some outside soils and lessening immediate evaporation of volatile chemicals.
Carefully remove your PPE. The next step is to carefully remove your gear. Remember that while some of the outside contamination may have been removed, simply using your bare hands to take off your gear unassisted is likely to transfer contamination to other parts of your body. Having someone assist you in doffing contaminated gear greatly reduces the chances for cross contamination.
Bag and isolate your gear. At this stage, only some of the contamination has been mitigated. Therefore, after you remove your gear, place it in an airtight bag or other container. Avoid having contaminated gear in the apparatus or vehicle occupant spaces unless you can isolate the exposure hazards.
Address personal hygiene risks. Additional steps not necessarily PPE-related include using absorbent wipes to clean skin areas that are most exposed, changing out of station/work uniforms and under clothing (which are also contaminated), and taking a shower. These are also active steps that you can complete at the emergency scene or definitely on arrival at the station to further control contamination.
Understandably, these procedures represent the ideal circumstances for controlling contamination found on PPE. For a busy department with limited resources, the described approach represents significant challenges to operational demands. Not all organizations have second sets of turnout clothing or spare gear. Cold weather and other response conditions can complicate the ability to carry out certain steps. Nevertheless, the key is to take some action to lessen your overall exposure to contaminants. Just getting your gear off, bagging it, and keeping it out of the apparatus cab goes a long way toward reducing further contact with hazardous substances. Many departments are already devising ways to meet the general objective of controlling contamination at the scene relative to their PPE; additional solutions are expected in the near future.
Even if you have been properly wearing your gear, you cannot assume that your skin and body were not exposed to harmful substances on the fireground. Fire smoke presents a complicated mixture of toxic chemicals, some of which are persistent and some of which are known carcinogens or acute skin hazards. Many of these chemicals will become entrained within the clothing materials or penetrate the clothing and get onto your skin. While your skin can be a great barrier for many substances, some chemicals more easily permeate and can get into your bloodstream more readily than others. Such chemicals with continued exposure over long periods of time can result in increased risk for a variety of health disorders. Therefore, an important practice includes effective contamination control while still on the fireground and isolating gear for full cleaning and decontamination.
#4: Properly Triage PPE Cleaning and Decontamination
The fire service is starting to come to grips with the fact that dirty clothing is no longer acceptable. Although at one point in the not so distant past, heavily soiled firefighter clothing was perceived as a badge of honor, showing the experience of the firefighter, such practices now show ignorance for the fact that wearing contamination is only unnecessarily and chronically exposing yourself to dangerous substances as well as transferring that contamination to those around you.

(2) Firefighter clothing becomes easily contaminated and retains that contamination if not properly washed; if soiling is visible, it is likely that the gear is still contaminated. (Photo courtesy of Gear Cleaning Solutions.)
While the recognition that structural fires are contamination events is increasing throughout the fire service, practices for properly cleaning that clothing still lag far behind. Over two decades ago, the Fire Industry Education Resource Organization (F.I.E.R.O.), with the support of S.A.F.E.R. (Southern Area Fire Equipment Research), NAFER (Northern Area Fire Equipment Research), and CAFER (Central Area Fire Equipment Research), established the first PPE industrywide guidelines for PPE cleaning that became the basis for NFPA 1851. NFPA 1851, first published in 2001, has subsequently been updated twice, but in all that time few changes have been made for dictating how turnout clothing should be cleaned.
The current and past editions of NFPA 1851 establish three levels of cleaning—routine, advanced, and specialized.
Routine cleaning is cleaning that takes place at the scene of the emergency incident. It has also been associated with machine washing and washing of clothing in a utility sink.
Advanced cleaning is machine cleaning with some specification for how this cleaning has to be performed. For example, wash water and drying temperatures cannot exceed 105oF, detergent pH cannot exceed 10.5, and the acceleration of the washer cannot exceed 100’G. Current procedures permit both conventional home laundering machines and more sophisticated and (usually) programmable washer/extractors. Advanced cleaning is required to be undertaken at least on an annual basis but is supposed to be applied whenever the conditions of soiling and use dictate a more thorough cleaning.
Specialized cleaning is relegated to any unusual contamination of the clothing elements that takes place. Although NFPA 1851 implies that this cleaning is for hazardous chemicals and certain other dangerous substances, no specific procedures are provided within the standard. Many fire departments simply do not know what specialized cleaning refers to, believing it to be needed when clothing has to be sent out to an ISP for cleaning.
The committee responsible for NFPA 1851 is working toward modernizing the requirements in the standard as they apply to cleaning. More importantly, the committee is working on changes to the standard that will provide a logical progression for how cleaning is undertaken and create a decision-making process for determining whether clothing can be adequately cleaned and what procedures are necessary. The new procedures are aimed at reducing the risk of continued contamination exposure from soiled and contaminated gear. The favored approach includes the following:
Brush/rinse, isolate, and bag the PPE at the scene. In the new proposed scheme, all cleaning and decontamination are preceded by preliminary exposure reduction procedures, which encompass the steps prescribed in the previous section for staying on air and applying techniques to reduce surface contamination, then isolating and bagging gear, preferably on the scene or as soon as possible.
Apply advanced cleaning to all fireground soiled or contaminated PPE. If turnout clothing is subjected to fireground exposures where it is soiled or contaminated, then advanced cleaning should be used for removing contamination. Even clothing that is soiled and not contaminated can still pose risks to the wearer where the clothing becomes more easily contaminated in subsequent uses.
Use sanitization for bloodborne pathogen and other microbial exposures. If clothing and equipment are contaminated with blood, body fluids, or other known biological contamination such as flood water, then the clothing must be sanitized or disinfected before and during the cleaning process. In general, sanitizers and disinfectants kill microorganisms but do not remove soils, so cleaning is also necessary.
If PPE can be decontaminated, use specialized cleaning for hazardous materials exposures. Any turnout clothing used in a hazmat incident or exposed to bulk chemicals on the fireground should first be assessed by the hazmat team or other knowledgeable authority who can determine whether the gear can be cleaned and, if so, can subject the gear to an appropriate set of cleaning procedures, usually specialized cleaning, that may involve additional pretreatments, special cleaning agents, or other techniques specific to the type of contamination.
Within the span of fireground exposures, certain contaminants commonly encountered also warrant specific decisions for cleaning or treatments. Asbestos is one such contaminant. You can encounter it on the fireground more frequently than you think, and it often isn’t identified until after the incident. Given the elevated rates of mesothelioma, a type of cancer related specifically to asbestos exposure, addressing PPE suspected of being contaminated with asbestos requires careful consideration. Although nondestructive testing may show removal of asbestos from gear, uncertainty often remains about the ability to put exposed gear back in service.
Surprisingly, bedbugs, although not acutely hazardous, are also a form of contamination requiring special treatment that often includes applying extreme temperatures for killing the insects and their eggs. Other emerging hazards, such as fentanyl and other opioid drugs where firefighter responses may result in exposures to powder, require decontamination techniques to avoid exposure to airborne drug powders.
Overall, the triage approach to making cleaning and decontamination decisions works to apply cleaning for any soiled and contaminated gear and ensure that the correct cleaning methods are used. For the most part, PPE subject to structural and other fires can be taken care of through advanced cleaning, but in several circumstances departments also have to anticipate the need for specialized cleaning or decisions where the clothing cannot be returned to service.
#5: Apply Appropriate Cleaning Methods
It would seem that once the department got to the step of actually cleaning the gear, all contamination issues would be easily resolved. After all, how hard is it to wash clothing? Yet, we now know that cleaning highly specialized clothing that can cost as much as $2,000 for a set including a coat and pants must be done properly. If not, the clothing does not become clean or, worse, cleaning performed incorrectly can actually damage the gear or diminish the service life of the clothing.

(3) The selection of the appropriate washer/extractor, detergent, and wash cycle are all important for the effective cleaning of turnout clothing. (Photo courtesy of Gear Cleaning Solutions.)
The chemistry of soil removal is relatively well studied. A lot of this science has come from commercial and domestic laundry practices that have been developed over decades as machines, detergents, and processes have evolved. Yet, much of the work that has gone into being able to get clothing and other textile products clean has focused on the removal of conventional soils and other common substances. The contamination from structural fires is much different. First, the smoke particles generated by a fire are generally very small, many in the range of a hundredth of a micron in diameter. Second, these particles are present in very high concentrations and, given their size, easily penetrate small pores and openings in the clothing as well as coat exposed surfaces. Third, the carbon particles adsorb fire gases created by the decomposition of materials during a fire, leading to a myriad of different chemicals. This adsorption holds onto the particles and the gases that otherwise would dissipate and be diluted in the environment. Added to these attributes are high levels of heat and moisture, which exacerbate the contamination process—especially when there are additional exposures to a variety of melted materials that can deposit on clothing surfaces. The result is a relatively complex set of contaminants that are distributed in different ways throughout the clothing. Moreover, given that no one fire is like any other fire, the vast range of conditions leads to varying degrees of contaminants.
Add to this the complexity of the clothing itself. Turnout clothing is not made from just one fabric; it also includes a variety of materials to make elements like trim, wristlets, reinforcements, integrated drag rescue or fall protection devices, and hardware. These materials all have characteristics that do not clean up the same way. Many in the fire service are often surprised about the restrictions placed on laundering gear, which are intended to protect the gear from adverse exposure circumstances, particularly that it has to be handled “gently” or the gear may be damaged by washing. These restrictions come in the form of low wash temperatures, no use of bleach, low alkaline detergents, and limits on drum spin acceleration. More sophisticated wash cycles need to be applied to ensure that maximum wash effectiveness is achieved.
Obviously, conventional washing in standard domestic machines is not going to work well. Instead, gear washing should include the following steps:
Properly prepare the clothing for washing. For turnout gear, separate outer shells from liners. Shells are generally much dirtier than liners, and the hardware on shells can damage the liners during washing. In some cases, protective hoods and textile components of helmets can also be cleaned with clothing under shells. Individuals handling contaminated clothing must be properly protected with wearing gloves, aprons, and sometimes a face mask or disposable respirator.
Use a sanitizer or disinfectant if needed. Clothing that is contaminated with blood or other body fluids or that came in contact with flood water will need to be at least sanitized, which means bringing the microbial contamination to a safe level. It is important to use only sanitizers or disinfectants that have been registered with the Environmental Protection Agency (EPA) or apply full cleaning processes that are known to be effective in removing biological contamination.
Use the correct machine. Do not use conventional washing machines for cleaning turnout gear because the center agitator will damage the clothing and will not result in efficient distribution of wash water and chemicals. Instead, employ a front-loading washer/extractor that is capable of being programmed and specifically set up to wash turnout clothing. These machines carefully control the water temperature levels and use an action of lifting and dropping the clothing inside the washing drum to provide effective detergent and water contact with the clothing. Machines also have an extraction cycle, which pulls much of the water out of the clothing. Many departments opt for 40- to 60-pound machines that provide the capacity for washing several sets of clothing at one time. The use of the machine requires a dedicated space in the station or another facility with access to ample hot water and the capacity to accommodate large quantities of rapidly draining rinse water.
Identify an appropriate detergent. A variety of detergents are on the market. Some suppliers advertise cleaning agents specific to firefighter protective clothing. Nevertheless, it is important to be wary of all claims. In some cases, a chemical supplier will offer a department a free automated dispensing device that integrates with the washer/extractor as an inducement to order chemicals. It is important to avoid general industrial chemicals that include high-alkalinity detergents (those with pH greater than 10.5 on their Safety Data Sheet), “sours” that reduce wash water alkalinity, and other cleaning chemicals that are not demonstrated for use with turnout clothing. Many gear manufacturers provide specific recommendations for their products.
Apply a comprehensive wash cycle. The wash cycle is the series of actions that the machine undertakes to clean the gear. It is often referred to as a “formulation,” and it includes filling the machine, agitating the clothing, injecting wash chemicals, and providing rinses. The washer/extractor controls the temperature and the length of time for each step. The majority of effective formulations involve at least three rinse steps and often include more than one detergent step. Each detergent or rinse step is usually followed by an extraction where the water is rapidly drained. Many machine providers have preprogrammed formulations that also address heavy soiling or provide separate wash cycles for liners as compared to shells. Also important in the wash cycle is the amount of clothing. In most cases, 80 percent of the machine capacity is used. Therefore, a 40-pound machine will allow for 32 pounds of clothing.
Dry clothing in a drying room or drying cabinet. Turnout clothing is best air dried, but this takes much longer than a dryer. Like conventional washers, dryers can sometimes quickly damage turnout clothing. In addition, earlier research from the late 1990s showed that ambient air drying is more effective as part of the cleaning process for PPE. The preferred method is to use an area set aside as a drying room where slightly elevated air temperatures can be blown over the clothing; alternatively, some machine providers also offer drying cabinets that accomplish the same thing but in a more limited space.
Inspect clothing after cleaning. Anytime PPE is cleaned, inspect it before putting it back in service. Make sure the PPE is fully dry and free of damage that could compromise its performance in reuse.
There are several variants to the cleaning process. Sometimes the clothing has stains that require pretreatment. Some processes use highly sophisticated machines or specialty additives such as ozone for sanitization purposes for biologically contaminated gear. Some forms of contamination warrant completely different cleaning or decontamination approaches as mentioned in the previous section. In general, many manufacturers offer guidance on how to properly clean their products. It may be necessary to contact industry experts to determine the appropriate cleaning/decontamination approach for controlling PPE contamination.
Many organizations turn to ISPs that provide a combination of cleaning, inspection, and repair services for firefighter PPE. NFPA 1851 has procedures for verifying their competence, primarily for inspection and repair services, but this is a program by which the ISP is regularly audited and reviewed. ISPs can be an additional resource for not only regular advanced cleaning but also specialized cleaning, depending on their capabilities. Many ISPs provide training for fire departments and other organizations in the proper cleaning and inspection of turnout clothing.
Improvements in the level of detail and the specifications for advanced cleaning and other forms of decontamination have been proposed for the upcoming revision of NFPA 1851. Expect to see additional requirements addressing not only turnout clothing but also the cleaning of other items such as helmets, hoods, gloves, and footwear.
Ultimately, within the next couple of years, new practices will be introduced that allow assessments for cleaning effectiveness, which will reinforce keeping gear clean. Although many questions still remain to be answered, the good news is that the fire service now appreciates clean gear and will look toward keeping it clean as one way of preventing unnecessary and preventable continued exposure to contaminants.
#6: Assess and Validate Cleaning Effectiveness
Despite acceptance of the premise that more cleaning is necessary for clothing on the fireground, the missing element is judging just how effective that cleaning is. We know that washing generally appears to remove soils and that clothing can look closer to its original appearance after being cleaned. In some cases, the clothing smells “clean,” leading us to believe that washing the clothing really works. Of course, there are those situations where difficult-to-remove stains remain, and many times older, frequently cleaned turnout gear looks dingy and faded, but we assume that most of the contamination is gone. Hopefully, we are right. Our research has identified several important findings.
Don’t rely on overly simplistic methods of determining PPE cleanliness. The complexity of turnout clothing contamination dictates that conventional methods for assessing cleanliness may not be accurate. Appearance and odor, while telltale signs of continued soiling, are not enough to assess whether the clothing has been adequately cleaned and, more importantly, decontaminated. Similarly, simple tests such as pressing a clean white textile or piece of paper against the clothing can only tell you if the item has no visible soil; it can’t tell you if the clothing is not contaminated. And while some cleaning processes impart fragrance to the laundered item, these odorants may only mask any scent from a continuing contaminant. The reality is that the smell of “clean” is no odor at all. The problem with determining that turnouts are clean is being able to evaluate the clothing without affecting its reuse. Certainly, samples can be taken from a clothing item and, using established analytical methods to reveal any potential lingering substances of concern, can be extracted and evaluated, but the trick is to be able to do this without taking the sample (and damaging the gear).
Figure 1. Cleaning Verification Kit Process
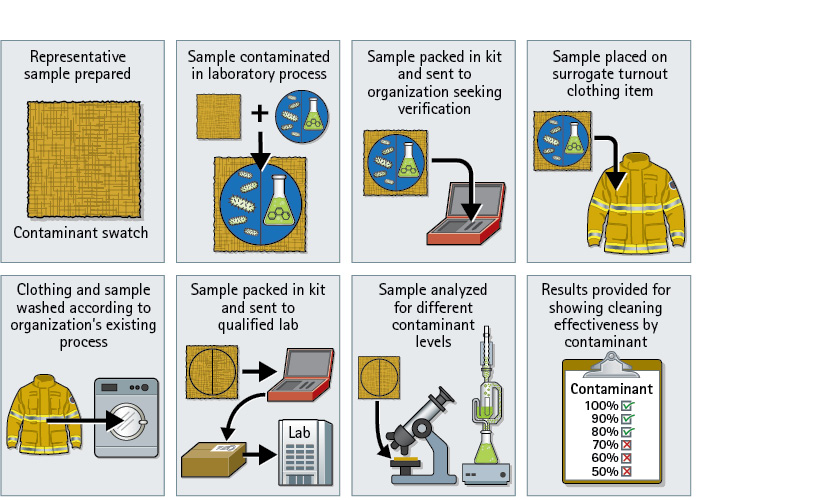
Steps in the proposed process for verifying that turnout clothing is cleaned of harmful contaminants. (Courtesy of author.)
Realize the limitations of nondestructive PPE sampling and analysis. Attempts to apply nondestructive techniques for analyzing clothing are fraught with difficulties. Some of us would like to think that it is just a matter of buying the right detector, pointing it at the clothing, and getting a reading that lists all of the contaminants. As much as we would like to believe that this all-capable instrument exists, it doesn’t. What’s more is that even if you were able to analyze the air space next to the clothing, the chemicals you find would not be the ones that you should have long-term concerns about. Any chemical that is easily released back into the air under ordinary conditions is volatile, and these chemicals will dilute out of the clothing relatively quickly. Instead, the problem lies with the chemicals that don’t evaporate easily, as those are generally the heavier, more complex chemicals—several are linked directly to chronic health conditions or cancer.
The ability to sample the clothing for identifying and quantifying these chemicals without permanently affecting the clothing is a significant technical challenge. First, sampling one area of clothing may not be representative of all areas of the clothing or all PPE that was exposed if the sample area and number of samples are limited. Second, analyses must be very specific and sensitive and provide meaningful results. Nondestructive sampling works well on certain contaminants. For example, it is possible to use specialized equipment to vacuum and capture nearly all particulates off the surface of clothing. Standardized industry methods can then be applied to identify and analyze the number of particulates—say, hazardous asbestos fibers—that remain on the clothing. Of course, as with any analytical procedure, there remains some uncertainty. For instance, when asbestos exposures clearly occurred and no asbestos is found, it is extremely difficult to rationalize results that do come back showing the presence of a few fibers still on the clothing after cleaning.
Exercise care in interpreting results from the analysis of cleaned clothing. One approach to evaluating clothing for contamination or cleanliness involves getting “samples” from the clothing and then sending those samples to a qualified laboratory for analysis. This requires a certain level of expertise. Sampling methods include using surface wipes, soaking the clothing in small volumes of water with detergent, and physically removing samples for destructive analyses. These techniques can easily be applied anywhere but have to be properly administered to get appropriate results. For example, in using a wipe, a relatively large area needs to be sampled to get enough contaminant to be able to analyze. Our research showed that pre-moistened laboratory wipes normally used for lead sampling are effective in capturing heavy metals like antimony, arsenic, and chromium. Yet, similar alcohol-based wipes don’t always do a good job of picking up the low-volatile compounds (like certain PAHs) and will only pick up surface contamination.
To capture the lower-volatile chemicals, which tend to be persistent in clothing, one of the turnout clothing cleaning companies has suggested using a soak tank with a low volume of liquid. This approach works to a degree, but the contaminants can be significantly diluted in the soak liquid, and it can be difficult to isolate substances of interest unless the analysis methods are fine tuned. This is important because most laboratories are used to evaluating soil, water, and air samples, not turnout gear. Moreover, even clean gear has chemicals in it that include dyes, finishes, and body residues that have to be sorted in any type of complete analysis. If removing samples from clothing for analysis, the greatest success occurs when target substances are identified. Otherwise, analyzing clothing for general contamination levels can be expensive and yield results that are difficult to interpret without defined levels of acceptable contamination.
To overcome the problems with directly sampling contaminated or cleaned gear, the Fire Protection Research Foundation project is in the process of finishing its work toward creating measurement tools that will allow us to determine how well any cleaning process works in removing key contaminants. The project is based on developing a methodology that can be applied anywhere for assessing whether key fireground contaminants have been removed by whatever machine, detergent/cleaning agent, or process in a way that provides a clear indication of decontamination effectiveness. The concept has involved developing a means for contaminating clothing material swatches uniformly in the laboratory with a set of representative substances; inserting these swatches into surrogate full clothing items; and then washing the full clothing using whatever cleaning procedures would normally be applied, including any pretreatments, special cleaning agents, and drying procedures. The previously contaminated material swatches are then removed from the clothing and analyzed for contamination levels to determine how much of the representative substances have been removed.
To initially address the principal contaminants of concern, the project team has focused on a limited number of hazardous substances that include dangerous heavy metals; toxic and suspected carcinogenic low-volatile chemicals including PAHs, phenols, and phthalates; and certain bacteria for demonstrating sanitization. This approach for the verification of cleaning effectiveness includes several elements, described below.
- Reproducibly contaminate swatches with known levels of contaminant. The key part of this approach has been to undertake the laboratory contamination of material samples in a way that simulates how clothing is typically exposed, especially to the array of fire gas chemicals and carbon particles in smoke. Attempts to contaminate materials with controlled exposures to smoke from selected burning materials, while proving realistic, are extremely variable and don’t allow the necessary precision to consistently evaluate decontamination effectiveness. Instead, the project team has developed a variety of approaches, depending on the substance, that involve various ways of applying the chemicals or bacteria to the swatches in a reproducible way.
- Select representative materials for swatch contamination. Efforts have focused on cleaning the outer shell, but the project team has found differences in how outer shells contaminate given the type of materials, their construction, and finishes. An examination of liquid absorption and wicking characteristics for field vs. new clothing samples showed a range of outer shell fabric conditions where new fabric and even some washed but unused fabrics show relatively low levels of liquid uptake in contrast to used fabrics in older gear that more readily absorbed and wicked liquid. Given that the condition of the fabric is important to how it is contaminated, the proposed approach uses a couple of representative fabrics.
- Use accurate and sensitive sampling techniques/analytical methods to quantify contaminant levels. For each type of contaminant, specific extraction and analytical methods are applied to indicate the amounts of the chemicals or bacteria contained in the fabric. These techniques are set at relatively sensitive levels and are applied to both unwashed and washed samples to get an idea of the contamination levels. In the case of heavy metals, some metals are already present in material dyes and finishes that have to be subtracted to get a clear picture of what is removed. Overall, a decontamination efficiency value is reported for chemicals. Additional research is being done to determine the acceptance levels for residual contamination based on existing toxicological limits set for other products, where available. For bacterial contamination, procedures developed by the EPA for qualifying disinfectants and sanitizers are being applied. The measure here is that the reduction of bacterial counts in powers of 10 are reported and compared against already prescribed levels of required effectiveness.
- Apply conventional wash procedures to evaluate process effectiveness. It is important to be able to evaluate the wash process the way it normally works to instill confidence that the procedures measure the effectiveness for how turnout clothing will truly be cleaned. To this end, detailed procedures have been developed that involve setting the contaminated swatches inside open mesh pockets on surrogate clothing items. These clothing items are set together with other “ballast” materials that form the bulk of the load. Specific instructions have been created for putting together the wash load so that the organization performing the cleaning will load the machine in a prescribed way that allows for a degree of precision in how the testing is carried out. The cleaning facility is instructed to perform the cleaning according to its normal procedures to allow assessment of the overall efficacy of the decontamination/cleaning process.
This approach has been subject to a variety of validation checks to ensure that it compares with ordinary advanced cleaning processes applied by different organizations, and it relates to the types of practices normally applied by the fire service industry. Early findings have shown that some forms of contamination may be difficult to remove using conventional cleaning approaches, but the use of the kit also allows refinements of existing processes and investigation of new equipment and cleaning agents that will result in improved decontamination.
When completed and implemented, specific cleaning equipment, agents, and processes will be evaluated to determine which wash conditions have the highest efficiencies in removing specific types of contaminants that are known carcinogens or skin absorption toxins. Just as important, differences in cleaning parameters such as equipment type, wash temperature, and detergent chemistry can be evaluated to identify the optimum conditions for getting turnout clothing clean. The establishment of these procedures can further give validity to cleaning verification where it is being proposed that ISPs and manufacturers can be qualified as having appropriate equipment and procedures to effect turnout clothing decontamination in the same manner that these organizations are now verified for their repair capabilities.
Additional efforts have been made to investigate the effects of the cleaning processes on turnout clothing itself. After all, with the expectation that cleaning is applied more frequently, there will be the need to ensure that the cleaning itself does not degrade clothing prematurely. Generally, the means of monitoring the effects of repeated laundering on clothing performance properties that address the protective qualities of turnout clothing are required by the specifications in NFPA 1971. Although it seems difficult to accept, laundering can shorten the life of turnout clothing relatively rapidly and, in some cases, adversely lower its performance for some types of properties. This means that cleaning processes must be verified for their removal of soils and contamination and evaluated to show that unusual clothing degradation does not occur when the cleaning process is applied regularly.
Clearly, maintaining PPE in a clean condition is not the overall answer for minimizing firefighter exposures to carcinogens. These exposures occur in a variety of ways, and several steps can be implemented to control contamination and limit contact with persistent health hazards. Still, ensuring the removal of persistent contamination possibly linked to adverse health effects along with implementing practices for controlling contamination offer at least one avenue of dramatically lowering contamination risks to firefighters.
Jeffrey O. Stull is president of International Personnel Protection, Inc., which provides expertise and research on the design, evaluation, selection, and use of personnel protective clothing and equipment and related products to end users and manufacturers. He has conducted numerous studies for effectiveness and performance of protective clothing and equipment in a variety of applications. Stull is the past chairman of the American Society for Testing and Materials F23 Committee on Protective Clothing, a member of several National Fire Protection Association technical committees on emergency responder protective clothing and related equipment, a participant of the government’s Interagency Board for Equipment Standardization and Interoperability, and the former lead U.S. delegate to the International Standards Organization for Protective Clothing. Prior to forming International Personnel Protection, Inc. in 1993, he was president of TRI/Environmental, Inc. from 1988 to 1993, an organization that provided certification testing and conducted research projects related to protective clothing. His career in protective clothing began as a project officer for the U.S. Coast Guard Office of Research and Development from 1983 to 1988, where he headed several programs related to personnel protection. He has a master of science degree in chemical engineering from the Georgia Institute of Technology and a master of science degree in engineering management from the Catholic University of America. He received his bachelor of science degree in physical sciences from the U.S. Coast Guard Academy. He is well published on all areas related to protective clothing and equipment, with several peer review articles related to protective clothing performance. He is considered one of the leading experts in the field of personal protective equipment.

