When YOU AS A firefighter respond to a fire, the most obvious threats are from the flames and the smoke. However, the less obvious threat is from the chemicals released into the air during a fire. Some chemicals, such as carbon monoxide, can harm you immediately after exposure occurs, which can cause symptoms ranging from nausea to seizures or even death.1 Some chemicals, such as benzene, can also have delayed effects, which can lead to cancer if you are exposed to it over time.2 Although you can’t see these chemicals and you might not feel them affecting your body, scientists have come up with some creative methods to measure your exposures. Below, we will discuss some different types of samples that can be collected from firefighters and what kinds of information the samples can (and can’t) give you about your exposures.
Sample Types
Firefighters are exposed to many chemicals, but it isn’t practical to look for all of them. This article provides information to help select the best sampling method for the variety of chemical classes and scenarios firefighters face.
Once a study has focused in on a chemical or chemical class of interest, there are multiple ways to collect samples. Generally, we will consider two: external and biological (internal). An external sample is collected from outside the body (on skin, on clothes, or in air/water/soil). Biological samples such as blood, urine, hair, and exhaled breath are collected from the body. Table 1 includes some common external and biological sample types used in firefighter studies for personal exposures. The table provides brief information on how the samples are collected and the type of information they can provide.
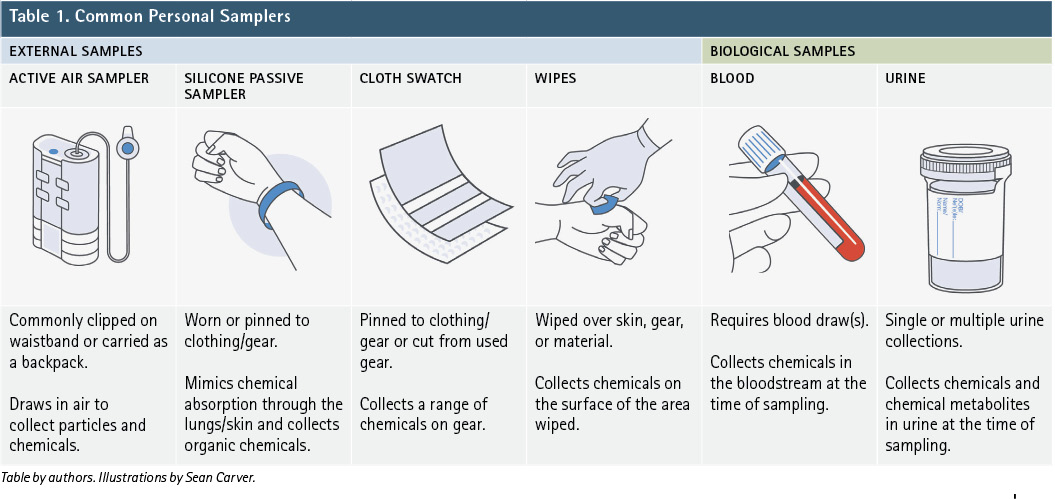
Beyond the sample types in Table 1, other tools to learn more about firefighting conditions and potential chemical exposures include questionnaires, GPS tracking, and personal logs. Recently, smartphone apps tracking personal activity and potential exposures have been released. Smartphones have also been connected to spirometers to measure respiratory health.3 These tools can be incredibly powerful, especially when combined with data from a physical sample.
Comparison of Sample Types
The different sample types each give scientists particular information and come with their own advantages and disadvantages. Table 2 breaks down some distinctions between the sample types. Shown in blue are logistical considerations that may make a sample type easier for firefighters to implement than others. The gray boxes indicate the types of samples that are best suited to look for types of contaminants.
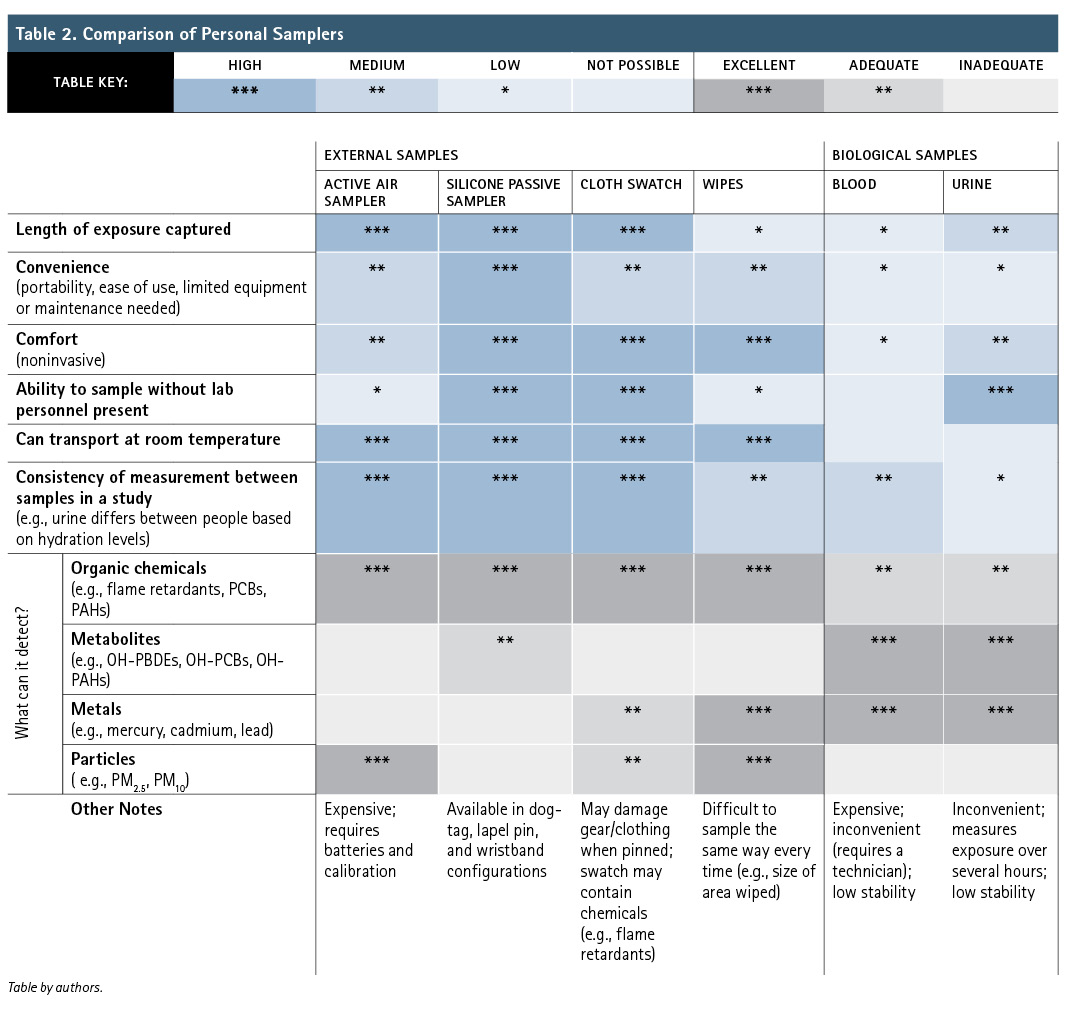
Considerations such as “comfort” for the firefighter from which the sample is being collected and “convenience” for both the collector and the firefighter providing the sample could be influential in deciding on a sample type. By electing to use a comfortable and convenient sample type, you could avoid adding stress or complications into already-demanding fire response situations. Similarly, sample collection can be easier to perform if it doesn’t require lab personnel to be present. For example, blood samples rank poorly in these practical categories because giving blood immediately after responding to a fire is painful, it is inconvenient to collect and transport, and it requires a lab technician to be present to draw the blood.
Consider the data quality—i.e., the collected data’s relevance to the study’s goals. For example, the “length of exposure captured” refers to the time window of exposure that the sample can represent. The active air sampler and silicone passive sampler absorb chemicals continuously over the entire period they are worn, spanning hours, days, or even weeks. In contrast, a wipe sample can only pick up chemicals that are on the surface when it is being wiped. Blood and urine samples identify what is currently in that fluid, so a single urine sample likely represents the previous six to eight hours of exposure, although some chemicals may be excreted much faster or slower than this. To more thoroughly capture the chemicals from an exposure, firefighters could collect all urine over a 24-hour period, which would require they carry a urine collection container with them at all times.
Another important consideration is consistency of measurement between samples in a study. Consistency can be high if the samples are collected the same way every time, but this is not feasible for all samples. For example, it is difficult to take a wipe sample the same way every time—the gauze would need to be wiped over the same area, with the same amount of pressure applied, and with the same pattern of wiping. For biological samples, everyone’s blood and urine will look a little different, influenced by factors like hydration and genetics. Fortunately, additional measurements can be taken to account for some variability in biological samples to improve consistency. Ultimately, consistency can make a study’s data more reliable and precise.
As mentioned earlier, the chemical of interest also influences the types of samples you should consider. For example, biological samples can be analyzed for evidence of chemicals that have been absorbed into the body. Scientists may look for a chemical directly, but often it is more appropriate to look for metabolites of the chemical in urine (what the original chemical becomes as a result of metabolism in the body). Hence, in Table 2, urine is marked as “excellent” for detecting metabolites but less so for the original organic chemicals that would be present in the environment. Additionally, some sample types can be used to detect soot and other particulate matter or metals, while others cannot. This table doesn’t cover all applicable considerations for choosing a sampler, but it provides a broad overview of the options.
Case Study: Choosing the Right Sampler Type
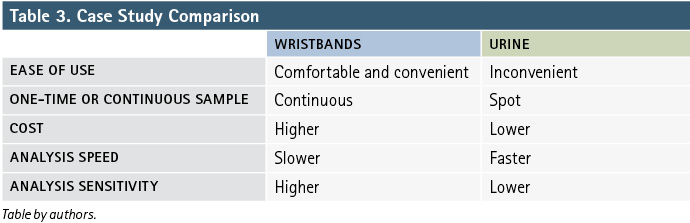
As an example, we will discuss results from a case study in which two types of samples were collected from structural firefighters: silicone passive samplers and urine. Silicone samplers can absorb a variety of organic chemicals from the air and skin. Table 2 shows that silicone passive samplers rank high in comfort, convenience, and the ability to sample without the aid of lab personnel. They also capture exposures for as long as they are worn.4 In contrast to the wristband, urine samples can be more demanding in terms of collection. You could imagine that wearing a wristband while on duty would be more comfortable and convenient than giving a urine sample immediately following a fire.
In this study, firefighters in Massachusetts, Florida, and Arizona contributed urine and wore silicone passive samplers to investigate exposure to a class of chemicals called polycyclic aromatic hydrocarbons (PAHs). PAHs can be produced during fires, and some PAHs are known to cause cancer. Both types of samples were collected while on duty, once when not on an active fire (a “baseline” sample) and for several fire responses. Silicone wristbands were worn underneath turnout gear during these fire responses, while urine was collected at several time points after each fire, each time point representing a unique sample.
Although both sample types were collected from the same firefighters around the same incidents and were looking for similar chemicals, there was a major difference in the results: The wristbands’ analysis showed PAHs to be present, while the urine samples’ analysis showed no evidence of PAHs.
Several factors might have contributed to this difference in results. One factor is that the length of exposure captured by the wristband and urine samples differed, which affects the concentrations of PAHs that end up in the sample. If concentrations in the sample are too low, chemicals may not be detected in analysis. In this case, each firefighter provided multiple urine samples from several time points after the fire. Each of those samples only contained a fraction of the total PAHs making their way through the body after a fire.
By comparison, the wristbands were capturing PAHs the entire time they were worn during the fire. One thing that may have improved the urine results would be if the urine samples from different time points had all been mixed together into a single urine sample for each firefighter. This mixed sample would have contained all of the PAHs and metabolites excreted in the urine after the fire rather than just portions and may have increased PAH concentrations to a detectable level in analysis.
Analysis Type
The type of analysis is also very important. The analysis chosen for the wristbands was more time consuming and expensive than the analysis chosen for the urine, but it also had a higher sensitivity. An ELISA analysis chosen for the urine samples was less sensitive and far less specific; it could tell you if PAHs and metabolites were present but not which ones. The ELISA analysis looked for PAHs and metabolites because we know that there are greater concentrations of PAH metabolites than of the original PAHs in urine.5 The low sensitivity of the ELISA means that PAHs and metabolites may have been present in the urine but concentrations were too low to detect.
Urine samples can be very useful in investigating exposures, but in this case, using the cheap and fast ELISA analysis on the samples did not show the firefighter PAH exposures that the wristbands were able to capture. Table 3 summarizes the differences between the wristbands and urine in this case study.
Key Questions and Considerations
Firefighters respond to calls that expose them to a variety of harmful chemicals. If we hope to address the health threats these exposures pose, a critical first step is accurately measuring the chemical exposures. This requires thoughtful collection of an appropriate sample type.
The success of a study of firefighter exposures relies heavily on forward thinking and planning. In the planning stage, there are several questions worth asking:
- Is the sample type appropriate for the question that the study intends to answer?
- Is the sample type logistically practical to collect? For example, if you want data from a blood sample, is it feasible for subjects to give blood directly after responding to a fire? This requires creating a sterile environment, paying a trained phlebotomist to draw blood, and the discomfort or physical stress for the firefighter giving blood.
- Is analysis sensitive enough for the samples? As in the case study, you must consider whether an analysis can detect the amounts of a chemical expected in a sample.
- What are the capabilities of the laboratory that will be performing the analysis? Different labs have different associated costs, processing times, detection limits, and resources for communicating results back to firefighters. It is important to know what to expect ahead of a study.
In firefighter sampling scenarios, scientists and firefighters rely on each other heavily. Scientists rely on firefighters to comply with the sampling procedures for the data to make meaningful conclusions. Firefighters rely on scientists to design a good study and inform them about their personal exposures. Therefore, it benefits everyone to make an informed decision on choosing a sample type that is practical for firefighters and can produce meaningful data with the appropriate analysis.
References
1. International Association of Fire Fighters, Carbon Monoxide Poisoning and Fire Fighters, 2020, Washington, DC: AFL-CIO.
2. American Cancer Society, Benzene and Cancer Risk, 2016.
3. Ping Zhou, Liu Yang, and Yao-Xiong Huang. “A Smart Phone Based Handheld Wireless Spirometer with Functions and Precision Comparable to Laboratory Spirometers.” Sensors, 2019.
4. Steven O’Connell, Laurel Kincl, and Kim Anderson. “Silicone Wristbands as Personal Passive Samplers.” Environmental Science & Technology, 2014. Washington, DC: American Chemical Society,
5. Aramandla Ramesh et al. “Bioavailability and Risk Assessment of Orally Ingested Polycyclic Aromatic Hydrocarbons.” International Journal of Toxicology, 2004. New Delhi, India: Sage Publications.
Authors’ note: This article references research conducted by a team of researchers at Oregon State University (Kim A. Anderson, PhD; Diana Rohlman, PhD; and Carolyn M. Poutasse, PhD; Lane G. Tidwell, PhD; Peter D. Hoffman; Michael Barton) and the Center for Fire, Rescue and EMS Health Research at NDRI–USA, Inc. (Sara A. Jahnke, PhD; Walker SC Poston, MPH, PhD; Christopher K. Haddock, PhD; Brittany S. Hollerbach, PhD). Illustrations in Table 1 were created by Sean Carver.
The Food Safety and Environmental Stewardship program in the Department of Environmental and Molecular Toxicology at OSU is a chemistry laboratory specializing in passive sampling technology development and quantitative analysis of environmental samples.
The mission of the Center for Fire, Rescue, and EMS Health Research (CFREHR) is to understand and improve the health of first responders through systematic research and evaluation.
Emily M. Bonner is a toxicology PhD student at Oregon State University in the Department of Environmental and Molecular Toxicology. She served as a Type II wildland firefighter (Pike National Forest, Colorado).
Kim A. Anderson, PhD, is the director of the Food Safety and Environmental Stewardship program in the Department of Environmental and Molecular Toxicology at Oregon State University.

