By Maryon J. Williams Jr.
Scenario: A fire rescue is needed in an industrial building with a nonzero explosimeter reading. Do you go in?
Explosimeters, or combustible gas indicators (CGIs), are designed to tell you if the atmosphere is explosive, not what gas is present or what you should do about it. In other words, “If you don’t know where you are going, you won’t know when you get there.” Several recent articles have discussed the use of CGIs.1-5 This article will discuss how to interpret the flammability readings.
Of the many fire calls for gas leaks, too many have had devastating results.6 Most explosion reports do not provide the gas readings before the explosion. Members of my fire company who responded to a gasoline pipeline leak left a water pump station just before an electric heater in the station ignited the vapors. They did not use an explosimeter at the time, and I was a half mile away and observed the top of the flames at a 45° angle up from my position.
Sensors
Most four-gas monitors used by fire departments have catalytic bead sensors for flammable gas detection. The principle of the catalytic bead sensor was shown first in 1926. The sensor, or Pellistor (Figure 1), consists of a platinum wire that heats up to hundreds of degrees F in the presence of a catalyst and is part of a bridge circuit along with a reference wire (Figure 2). The sensor measures the flammability of certain low molecular weight flammable gases but does not identify the gas. The sensor needs oxygen to work so that if you lower your meter into a space with less than 10 percent oxygen, the readings will be inaccurate.
RELATED FIREFIGHTER TRAINING
- Four-Gas Air Monitors: A Survey of First Responders’ Competencies
- The Multigas Meter
- Hazmat Survival Tips: Interpreting Readings from Atmospheric Monitors
Other types of sensors are not so common in the fire service, but they are worth consideration. These include metal oxide semiconductor sensors for high sensitivity; nondispersive infrared detectors for single gases; tunable diode laser absorption spectroscopy sensors; and remote methane laser detectors, which work up to 30 meters. Photoionization detectors can detect heavy gases, while microelectromechanical systems sensors can classify gases into six categories.
A fire department needs a meter that will sense a variety of gases. Many volatile organic compounds (VOCs) are detectable with the catalytic bead sensor. Common VOCs are natural gas, propane, liquefied petroleum gas (LPG), and gasoline. Less common are acetylene and hydrogen. Gases that cannot be detected with a catalytic bead sensor include heavy fuel vapors such as diesel fuel and hydrogen sulfide (H2S), which is a poison to the sensor. Other poisons include silicone (tire shine products), tetra-ethyl lead (gasoline), and halogen compounds (fire extinguishers, refrigerants). Some meters have filters to prevent the poisons from getting to the sensors or a second bead to resist silicone poison. When high levels are detected, some meters shut off to save the sensor.
Response Criteria
The explosive span of a gas (Figure 3) ranges from the lower explosion limit (LEL) to the upper explosive limit (UEL). The LEL will decrease with increased pressure, temperature, or oxygen. For instance, a flammable gas is more likely to ignite in a room with an oxygen source than in a room with normal air. A distinction must be made between percent by volume, often used by utility workers who usually deal with one gas, and percent LEL, often used by firefighters who deal with many gases. Many department standard operating procedures do not give a level certain for evacuation of a building. Typical default settings are 10 percent LEL for low alarm and 20 percent LEL for high alarm.
The criteria differ among agencies as to when to evacuate. The Northeast Gas Association,7 which has a useful program for first responders, advises entry if the gas reading is “well below” the LEL. At least one utility8 requires evacuation of a building with any open-air methane reading above 1 percent by volume or 20 percent of LEL. National Fire Protection Association (NFPA) 329, Recommended Practice for Handling Releases of Flammable and Combustible Liquids and Gases,9 provides for evacuation at 50 percent LEL. NFPA 350, Guide for Safe Confined Space Entry and Work,10 says atmospheres that have reached 10 percent LEL are considered hazardous and requires additional precautions. The National Institute for Occupational Safety and Health (NIOSH)11 sets the value of the immediately dangerous to life or health (IDLH) at a minimum of 10 percent LEL for safety reasons. A flammable gas is not like a poisonous gas, which has a meaningful level for IDLH based on toxicity. Occupational Safety and Health Administration12 guidance says, “Atmospheres with a concentration of flammable vapors at or above 10% LEL are considered hazardous when located in confined spaces. However, atmospheres with flammable vapors below 10% LEL are not necessarily safe.”
Figure 1. Pellistor Gas Sensor

(Figure by Crowcon Detection Instruments.)
Figure 2. Bridge Circuit
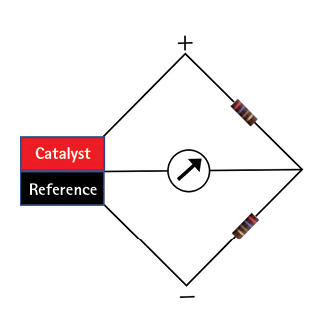
(Figures 2-8 by author.)
Calibration
A competency study of 185 firefighters13 found that only 37 percent could identify the calibration gas, only 72 percent knew to zero the meter in fresh air, and only 32 percent could identify the percent LEL alarm point. The study concluded, “First responders should receive more frequent training with regard to ambient air testing near an incident.”
A common calibration gas is methane, which has an LEL of 4.8 percent by volume so that 10 percent LEL is 0.5 percent by volume, or 5,000 parts per million. Normal air consists of 21 percent oxygen, 78 percent nitrogen, and 1 percent other gases (Figure 4). When you calibrate with methane, heavier gases read too low (Figure 5) because the heavier gases have more energy than the lighter gas.
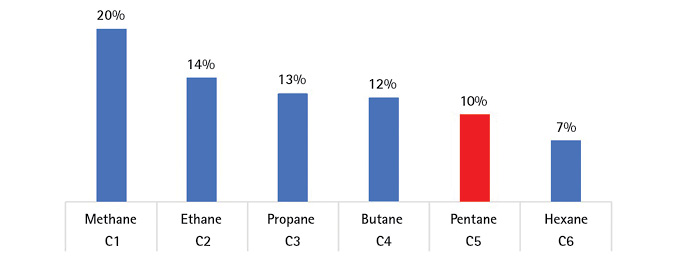
For instance, a typical meter calibrated with methane will read 5 percent LEL when exposed to 10 percent LEL pentane. If you calibrate with a heavier gas such as pentane, the lighter gases read too high. A meter calibrated with pentane (Figure 6) will read 20 percent LEL when exposed to 10 percent LEL methane. “Calibrated readings” can be twice over or half under actual values!
Figure 3. Methane in Air at 68oF and One Atmosphere

Figure 4. Components of Air

Many of the flammable gases a fire department will encounter are mixed gases whose composition can vary with the season and with the geographical area. The readings are inherently inexact, and the calibration factors are best provided by the manufacturer. In Figure 5, natural gas contains at least 75 percent methane (C1) and as much as 10 percent ethane (C2). LPG can be considered an average of propane (C3) and butane (C4). Gasoline is a complicated mixture of many hydrocarbons (C4-C12) for which one manufacturer14 gives a multiplication factor of 2.1 for a meter calibrated with methane. For sewer gas—composed of methane, H2S, gasoline, mineral spirits, and others—the meter is better used to determine where the gas is rather than how flammable it is. Precise readings can be made only with a single known gas. Even so, accuracy can be 10 percent of the reading—i.e., 10 percent LEL can be 9 or 11 percent.
Full calibration that involves changing the sensitivity of the meter should be done carefully to avoid introducing errors. The end user should perform a bump test frequently to verify that the meter will alarm. Most fire departments use a gas monitor so infrequently that bump testing a monitor just before use is going to take precious time. An industrial site that uses gas monitors might bump test daily. In one case, crews arriving to an industrial site incident used the site’s monitor because they knew it was bump tested that morning. In the incident critique, the chief said they should have used the department’s monitor. Also, fire department use of a calibration gas different from the site’s calibration gas has caused long delays while the department resolved the inconsistency.
Sensor Response Time
The catalytic bead sensor can take 30 seconds to several minutes to respond (Figure 7). The gas must first get to the sensor by diffusion, aspirator bulb, or pump. The time for gas to travel in a tube can take approximately two seconds per foot. Then consider the sensor rise time, which is the time it needs to get to 90 percent of the final value. (Note: Heavier gases take longer than lighter gases.) There have been cases where gases were sampled for only a few seconds and declared safe but all that was measured was the air in the sample tube.
Figure 5. Methane Calibration
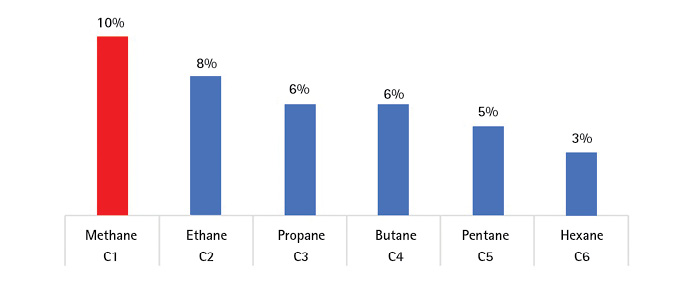
Figure 6. Pentane Calibration
Sample Location
Even if a gas sample is below LEL at one location, an explosive concentration can be at another. Gases can migrate horizontally through cable or pipe conduits to seemingly random areas. Sampling should be done throughout the space including possible sources and the ventilation system (Figure 8). Lighter-than-air gases include hydrogen, methane, and acetylene. Ethane is similar to air in density, and heavier-than-air gases are propane, LPG, and gasoline.
Figure 7. Response Time for Light Gas vs. Heavy Gas

Figure 8. Methane Is Lighter Than Air
Back to the Question
What do you do at an industrial building with a nonzero-percent LEL? Rarely can you decide to enter ahead of time. An explosion can occur when the gas is any level between LEL and UEL. The responder must make a critical decision based on the following factors in addition to the percent LEL:
- How critical is the emergency? (Is someone known to need rescue?)
- What gas is present? (There could be more than one gas.)
- Is smoke or fire showing? (If not, there could still be an explosive condition.)
- What was the calibration gas? (It is ideal if it is the same as the gas present.)
- When was the last bump test or calibration? (Ideally, just prior to the incident.)
- What is the calibration correction factor? (If the gas present is methane and the calibration gas was pentane, the meter is reading low. If the gas present is pentane and the calibration gas was methane, the meter is reading high.)
- Is there enough staffing available? (Both entry and standby are needed.)
- Is the utility representative on site? (He would have his own meter.)
- Is there a department policy? (If yes, the policy should be based on best practices for your department.)
Preplanning will give firefighters tools with which to make decisions even though the plan likely will change based on the situation at hand. The decision to enter an area is based on more factors than percent LEL. The decision is easier if the percent LEL is zero than if the % LEL is above zero. A meter calibrated with methane can give false low readings for heavier gases, while a meter calibrated with pentane can give false high readings for lighter gases. Now is the time to determine your policy for entering a potentially explosive space, the strengths and limitations of your explosimeter, and what flammable gases are in your district.
References
1. Giroux M. “What’s Your First Step in a Confined Space Rescue?” Fire Engineering, November 2020. Volume 173, Number 11, pg. 18-20.
2. Knapp J. “Tactical Size-Up of Natural Gas Emergencies.” Fire Engineering, November 2020. Volume 173, Number 11, pg. 33-40.
3. Leer J. “The Components of the Gas Detection Puzzle.” Fire Engineering, November 2020. Volume 173, Number 11, pg. 47-53.
4. Knapp J. “Tactical Size-Up for Explosive Gas Emergencies.” Fire Engineering, April 2020. Volume 174, Number 4, pg. 49-56.
5. Knapp J and B Focht. “Tactical Procedures for Explosive Gas Alarms.” Fire Engineering, May 2020. Volume 174, Number 5, pg. 33-41.
6. “FDNY Battalion Chief Killed When Suspected Drug Lab Explodes,” September 2016, https://emberly.fireengineering.com/leadership/fdny-lodd/#gref.
7. Northeast Gas Association, First Responder Training, Natural Gas Pipeline Safety.
8. PSE&G Emergency Responder Program Review [2012].
9. NFPA 329, Recommended Practice for Handling Releases of Flammable and Combustible Liquids and Gases, section 5.4.5.1. [2020].
10. NFPA 350, Guide for Safe Confined Space Entry and Work, section 6.3.5.3.2. [2019].
11. NIOSH. Current intelligence bulletin 66: derivation of immediately dangerous to life or health (IDLH) values. Cincinnati, OH: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication 2014–100. [2013].
12. OSHA. “Precautions and the order of testing before entering confined and enclosed spaces and other dangerous atmospheres.” 1915.12(b)(3) Appendix.
13. Handy R. “Four-Gas Air Monitors: A Survey of First Responders’ Competencies.” Fire Engineering, Volume 168, Number 11, pg. 53-61.
14. mPower Electronics. “TA-7 Combustible Gas Sensors.”
Maryon J. Williams Jr., Ph.D., P.E., is a 20-year veteran of the volunteer fire service, a captain of a high-angle rescue team, and an emergency medical technician. He has degrees in electrical engineering from Georgia Technical Institute and Rutgers University and has held teaching positions at Rutgers University and the Medical College of Georgia. As a consultant, Williams surveyed hospitals for nitrous oxide and ethylene oxide. He is also a practicing engineer with Condition Analyzing Corporation engaged in surveying ships and has authored several articles for Fire Engineering.

