By Kristina Kreutzer
The key to the safe resolution of any incident is a proper size-up. If officers understand the environment, the building, the building contents, and the fire conditions, they can make good tactical decisions. At a hazmat incident, understanding the nature of the chemical is much like understanding the behavior of fire. For a hazmat incident, you need to understand the environment, the containers, and the materials involved in the incident.
With a hazmat incident, it is generally not possible to see the problem, in contrast with smoke and fire at a fire scene. A hazmat technician or specialist is expected to use references to find the data that indicate how the material will behave. The question is, how do you easily and correctly translate those numbers into strategies and tactics?
Example: You are dispatched for a leak at a local university lab, which has been evacuated. Approximately one pint of material was in use, and you need to send in a team to find out exactly what is going on. The technical data on the material in use are as follows:
- Boiling Point (BP) = 78°F
- Freezing Point (FP) = 7°F
- Vapor Pressure (VP) = 630 mm Hg
- Specfic Gravity (Sp. Gr.) standard = 0.69
- Solubility (Sol) = 40% (solubility in water is implied unless indicated otherwise)
- Lower Explosive Limit (LEL) = 5.6%
- Upper Explosive Limit (UEL) = 40.0%
- Ionization Potential (IP) = 13.6 eV
- Permissible Exposure Limits (PEL) (Time-Weighted-Average (TWA) = 10 parts per million (ppm)
- Immediately Dangerous to Life or Health (IDLH) = 50 ppm
- *LC50 (40 min, rat) = 63 ppm [skin]
- **LD50 (dermal, rabbit) = 6.9 mg/kg
- **LD50 (eye, rabbit) = 1.1 milligrams per
kilogram (mg/kg)
*LC50 is the concentration of a chemical in a test atmosphere that kills half the test subjects within one hour when inhaled.
**LD50 is the dose that, when swallowed, injected, or applied directly, kills half the test subjects.
Organizing Information
How do you make use of the data for decision making? How do you determine the detection methodology? How do you decide what is the appropriate type of personal protective equipment (PPE) for this incident? How do you translate numbers into actionable items?
A good way to understand a complex problem is to systematically consider the pieces and how they are related. One way to organize hazmat/chemical information in a way that allows the user to make a quick interpretation for predicting the behavior of a given material is in Figure 1.
| Figure 1. Probability vs. Consequences of Contact Chart |
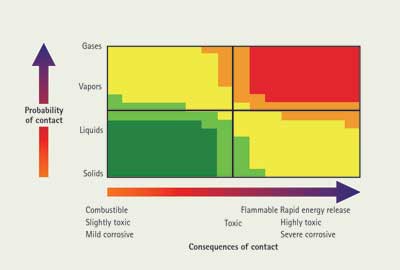 |
The two key parameters are the probability of contact and the consequences of contact with either the material or energy generated by the material. The probability of contact is an agglomeration of the physical properties of the material and its interaction with the environment. Materials are frequently encountered under conditions other than those listed in “the book,” so you have to consider the incident conditions. The other consideration is the consequences of contact, which relate to the chemical properties of the material. A given material may have multiple mechanisms of harm: energy releasing, corrosive, and/or toxic. Each of these can be plotted separately on the chart. Tactics can be used to address each of the problem areas until the safest point can be found.
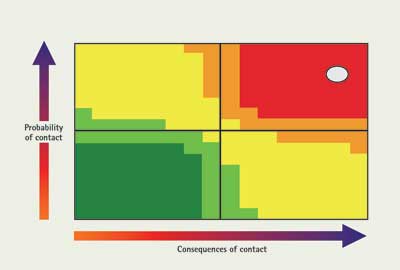
Consider a release of hydrogen chloride gas, which is a strong acid with the following properties:
- BP = -121°F
- VP = 40.5 atmospheres, unit of pressure (atm)
- Relative Gas Density (RGas D) = 1.27
- Sol = 67%
The material is a gas, so there is a high probability of contacting it during an entry, if unprotected. It is also a fairly strong acid, so there are some relatively bad consequences of contact. The dot is plotted in the upper right part of the chart in Figure 2. What can a responder do to mitigate this situation? You can address the probability of contact several ways. One is to use proper PPE to avoid direct contact with the material—in this case, an encapsulating suit because of the high probability of contact. Another way to avoid contact with a material, depending on the mission, is by detection. If the wet pH paper on a stick starts to turn red, you know there is acid, and you may be able to avoid that location. Another way to avoid contact with the material is material management. You can use its water solubility and absorb it with a fog stream. Another option is ventilation. These actions serve to decrease the probability of contact, as shown in Figure 3.
| Figure 2. Consequences of Contact: Hydrogen Chloride Gas |
 |
| Figure 3. Hydrogen Chloride Gas – Effect of Tactics on Material Behavior |
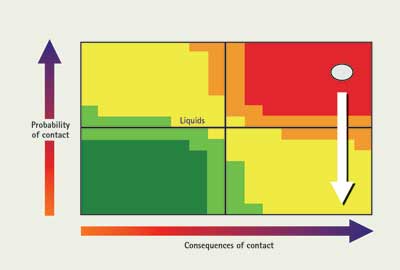 |
On the other hand, it is possible to neutralize an acid by contacting it with a base. Since the acid is being neutralized, the consequences of contact are diminishing, and the dot would move to the left on the chart. If this option is selected, note that the neutralization reaction normally generates heat. In the case where a fog stream is applied to an acid cloud, the material is also being diluted, decreasing the consequences of contact. It is important to remember that pH is a logarithmic scale and that you will have to dilute something 10 times to change things one pH unit. In this case, the consequences will change but relatively slowly. Again, when a concentrated strong acid or base is dissolved in water, heat is generated. In some cases, enough heat can be generated to boil the water, which may cause the acid or base to splatter.
The probability of contact relates to how the material will move. In the example above, a gas expands on release and moves relatively easily, so there is a high probability of contact. Liquids and solids, with the exception of fine particles or droplets, tend not to move as easily as gases. Given the proper environment and energy input, they can be converted to an aerosol and move in a manner similar to a gas. An example of this is an explosion involving a sulfuric acid tank. Sulfuric acid has a VP of 0.001 mm Hg (a standard unit of pressure), which means that it will not move under normal conditions. That is the reason people are able to pour sulfuric acid into car batteries wearing protection such as eye protection and gloves. In the case of the explosion, the energy from the explosion can convert the sulfuric acid to a mist or an aerosol, which can easily travel and can easily be inhaled. A much different level of protection would be required to make entry into such a scene.
Note that the consideration is probability of contact and not something as easily measured as VP. VP is certainly a consideration, but it is one of many considerations that make up the probability of contact. Understanding the numerical data, the environment of the exposure, experience with similar situations, and good judgment are all parts of estimating the probability of contact with a given material.
Most first responders are not accustomed to translating a VP number into a description of how a given material will behave. A useful method to bring the number into the real, actionable world is to compare it to familiar materials and make a prediction of its behavior. The VPs of some relatively common chemicals are listed in Table 1.

Click to Enlarge
With an energy-releasing material, the probability relates to contacting the energy, not just the material. What is the likelihood of an energy release, and where will the energy go if it is released? This is much harder to predict. Again, training and experience are the keys to making a proper assessment. What is the probable radius of a fireball generated from the boiling-liquid, expanding-vapor explosion (BLEVE) of a railcar containing propane? Studying historical incidents is especially useful with this class of materials.
Energy-releasing materials include explosives, flammable gases, flammable and combustible liquids, flammable solids, spontaneous combustibles, oxidizers, radioactives, and materials undergoing an exothermic chemical reaction, the most common of which are the combustion and polymerization reactions. Protection and detection tactics must address the possibility of encountering energy, not just the material, when dealing with this class of compounds.
When considering how energy-releasing materials are different from the hazards presented by other materials, consider fire smoke and backdraft. Smoke is both a fuel and a collection of toxic materials. For the smoke to be a toxin, you must contact the smoke. The toxin must enter the body, typically by inhalation or skin absorption. You have to breathe or touch smoke to be harmed in this manner. However, under the wrong conditions of smoke, oxygen, and heat, there is a potential for backdraft, which presents a much different problem. In this case, even if firefighters are standing outside the building, out of the smoke, the energy released by the explosion can still come out and injure them. Energy travels away from where the material generated it, so it is important to avoid the material’s sphere of influence, not just the material. The history of line-of-duty deaths attributed to hazardous materials contains a very high percentage of incidents involving energy-releasing materials. Recognizing the hazard and staying away from the area where the generated energy may go are key to safely working with this type of material.
Corrosive materials include acids, bases, and some oxidizers. They can be found in Department of Transportation hazard classes 2, 5, and 8, although some class 4 materials may also generate acids or bases during their reactions with air or water. Corrosive materials can typically attack the skin and mucous membranes of living beings, and they may also corrode metal, including PPE such as self-contained breathing apparatus (SCBA).
Consider both the inherent corrosive strength and the concentration of the material. For example, a moderately acidic solution can arise from a dilute solution of a strong acid (sulfuric, hydrochloric, nitric, and so on) or a concentrated solution of a weaker acid, such as acetic acid. The effect of corrosive materials varies with concentration. In general, more concentrated materials have a greater corrosive risk, although there are some exceptions—for example, the action of sulfuric acid on metal, where intermediate concentrations may be worse than highly concentrated acid.
A hazardous atmosphere can come from either the presence of a toxic material or the absence of oxygen. A toxic material can cause harm, and toxicity refers to the amount of material needed to cause harm. Some considerations when evaluating a toxic hazard include the toxicity, the route of exposure, the dose (the amount that enters the body), the duration of the exposure, the sensitivity of the individual, and interactions with other substances. Some materials are toxic in very small quantities, so avoid exposure to parts per million (ppm) levels of these materials.
A variety of references contain information on the toxicity of materials. One method for estimating the relative toxicity of a material is to compare potential exposures during an incident to occupational exposure levels, such as those listed in the National Institute for Occupational Safety and Health Pocket Guide or similar references published by industrial hygiene organizations. Multiple organizations have published exposure limits (Table 2); but they can be grouped into everyday occupational exposure limits such as PELs (safe), ceiling, short-term exposure limits (STEL) (unsafe), and IDLH (dangerous). It is also useful to compare air-monitoring values obtained by an entry team with the occupational limits to better understand the risks to personnel. Other data that can be useful for comparison are lethal dose or lethal concentration data (Table 3), which can be found on some material safety data sheets. Although you should not enter a lethal atmosphere where a lethal exposure could occur if protective measures fail, you can still use the data to compare an unfamiliar chemical with better-known chemicals to predict material behavior.

Click to Enlarge
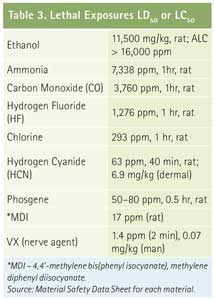
Click to Enlarge
When dealing with a hazardous material, the tactics for avoiding contact are typically detection, protection, and material management. Protection includes respiratory and skin protection—for example, chemical suits or turnout gear and SCBA. Detection is the electronic equivalent of warning properties: Can you see the material before there is enough material present to hurt you? Material management includes strategies to move or contain the material, including ventilation, damming, diking, and diversion. Material management may be a defensive operation.
Hazmat technicians need to be able to understand and use a detection scheme for the materials to which they respond. Detection equipment can be used according to the type of material in question. For energy-releasing materials, thermal imaging equipment can be very useful in finding the heat produced by the material (photo 1). A meter such as a four-gas meter with an LEL sensor or a combustible gas indicator is the main instrument used to find concentrations of the material that can catch fire or blow up.
 (1) The heat from a drum as seen with a thermal imaging camera. (Photos by author.) (1) The heat from a drum as seen with a thermal imaging camera. (Photos by author.) |
An important note is that if the person using the equipment is getting readings on the instrument, there is a possibility that the material may be within its energy-releasing sphere of influence—the released energy may be able to reach where the person is, much like a firefighter standing outside a structure when a backdraft occurs. Photoionization detectors (PIDs) may be useful for some energy-releasing materials. They are sensitive enough to give earlier warning that the material is present because they read in ppm instead of percent of the atmosphere.
Note that some energy-releasing materials such as small hydrocarbons (methane, propane being most common), methanol, acetonitrile, and hydrogen will not be indicated by the PID using the standard 10.6 eV lamp. For corrosive materials, pH paper and specialized detectors are the most common methods to detect a material. pH paper should be used frequently because it may allow the hazmat technician to find a corrosive problem before it damages other detection equipment.
A wide variety of detection equipment is available for finding toxic materials. The equipment cannot determine the toxicity of a given compound, but it can detect the ppm or part-per-billion levels that can be toxic. The PID is the workhorse instrument in this category; however, specialized detectors and colorimetric tubes can be used. Again, it is important to know what the equipment can and can’t see, along with the common cross sensitivities for a given piece of equipment. The detection plan generally uses multiple instruments to detect the various hazards that may be present.
PPE is generally the last line of defense in dealing with a hazardous material, but a hazmat technician may need to enter a spill area to do the job—for example, to plug a leak. Protection can be either skin protection or respiratory protection. In hazmat emergency response, the workhorse equipment is SCBA. When doing initial monitoring, SCBA is almost always worn because someone has to determine what the material is and how much is there before air-purifying respirators (APRs) can be used. For smaller spills, it may be faster to do the job using SCBA rather than acquire the proper cartridges. For longer-term operations, however, it may be advantageous to switch to APRs, as it leads to less physical stress on the entry team.
Skin protection can vary depending on the material, but it can still be broken down by type of hazard. If the hazard involves an energy-releasing material, fabrics such as Nomex® are highly beneficial. This can include turnout gear or specialized chemical resistant suits. If the material presents a corrosive or toxic hazard, suits with laminate materials are preferred. Note that a given suit fabric can be sewn into a variety of configurations—encapsulating level A suits for materials that have a high probability of contact, hooded coveralls, or other forms for situations with a lower probability of contact.
Each PPE manufacturer can give guidance to the preferred PPE for a given situation. There are Internet-based guides, cell phone applications, and manufacturer hotlines for PPE guidance. Again, note that the probability of contact may be different from the textbook behavior of the material depending on the environment, so take this into consideration when consulting the manufacturer for advice on suit selection. SCBA can be damaged by contact with a corrosive material, so it may also need to be protected by the chemical suit. For example, a single exposure to chlorine may be enough to cause visible damage to SCBA (photo 2).
 |
| (2) This relatively new SCBA connection was damaged by a single exposure to chlorine during a structure fire. |
One often overlooked aspect of skin protection is the gloves. The gloves are most likely to contact the spilled material. It is important to use glove charts and the correct glove for the given material. It may also be necessary to protect a chemical-resistant glove from thermal or especially physical damage. Overall, the PPE choice may be broken down by type of hazard present (suit material of construction) and the probability of contact (style of suit—encapsulating and so on).
Going back to the initial problem of the lab spill, how would you go about performing a reconnaissance entry? First, consider what the material will do under normal conditions. It wants to be a gas near room temperature, so there it will have a high probability of contact. It has two main hazards: It is very toxic, and it has a fairly wide flammable range. Based on this assessment, it can be placed on the chart as shown in Figure 4. The question is, how do we mitigate the problems?
| Figure 4. Charting Material from Example 1: Initial Conditions |
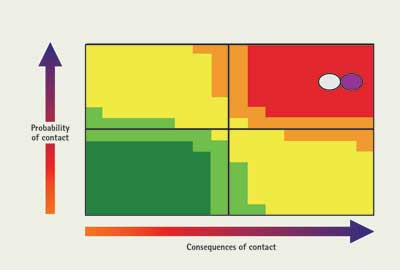 |
First, consider the toxic hazard. Can you avoid contact with significant amounts of the material in this mission? If you have a detector, you can stay out of toxic levels of material. In this case, even though a PID will not work, this material has electrochemical sensors and colorimetric tubes available, so there is a viable detection scheme for quantifying relatively small amounts of material. This will lower the probability of contact.
Also consider protection. This material can be absorbed by the skin, but if the mission is reconnaissance, contact with liquid is not expected. This material is toxic by inhalation, so SCBA will be used for respiratory protection. Both detection and protection work to lower the probability of contact with a toxic amount of the material (Figure 5).
| Figure 5. Charting Material from Example 1: Effect of Tactics |
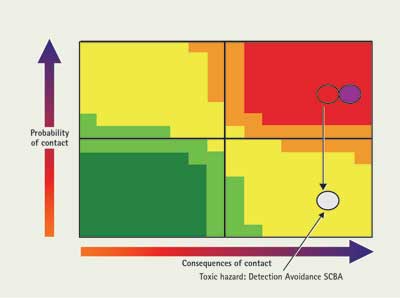 |
Consider also the flammable hazard. Although it is relatively easy to avoid contact with the material, it is not so easy to avoid contact with a fireball if the material finds an ignition source. Ways to decrease the fire hazard include securing potential ignition sources and ventilation. Given that the fire hazard may not be as secured tactically as the toxic hazard and it is possible to be within a material’s sphere of influence without contacting the material itself, turnout gear with SCBA would be a good choice for this particular mission.
Overall, systematically considering the probability and the consequences of contact with a given material breaks a more complex problem down into simpler components that you can more easily handle. Base protection and detection schemes on classifying the material by energy-releasing, corrosive, or toxic hazards and considering the mobility of the material.
Additional Links
Hazmat Survival Tips: Hazardous Materials Incident Size-Up
Hazmat Survival Tips: 10 Common Errors Committed During Incidents
Hazmat Survival Tips: 10 Initial Response Considerations for First Responders
KRISTINA KREUTZER, Ph.D., is a research chemist with DuPont, where she is an assistant chief with the site emergency response team. She is also an assistant chief with the Mill Creek (DE) Fire Company. She obtained her BS in chemistry from the University of Maryland and a Ph.D. in chemistry from the Massachusetts Institute of Technology. She is a nationally certified fire officer II and an instructor at the Delaware State Fire School.
Kristina Kreutzer will present “Hazmat: Initial Approach and Actions” on Monday, April 20, 1:30 p.m.-5:30 p.m., at FDIC International in Indianapolis.
More Fire Engineering Issue Articles
Fire Engineering Archives

