Chemical Data Notebook Series: Ethylene Oxide
HAZARDOUS MATERIALS
FEATURES
Ethylene oxide, a suspected carcinogenic, is a colorless liquid with a flash point of -4°F that is extremely volatile, producing tremendous amounts of flammable vapor. Its boiling point, 51°F, is so low that if unconfined, it will boil away rapidly at room temperature. Ordinarily, liquids have much higher boiling points, and remain in the liquid phase at room temperature, even though they may be evaporating fairly rapidly.
Since room temperature (72°F) is 21°F above the boiling point of ethylene oxide, some reference sources will list ethylene oxide as a gas. The very low boiling point and the fact that the vapors are flammable make ethylene oxide extremely hazardous. As we go through some of the chemical’s other properties, it will become apparent that ethylene oxide has many more hazards.
PROPERTIES/IDENTIFICATION
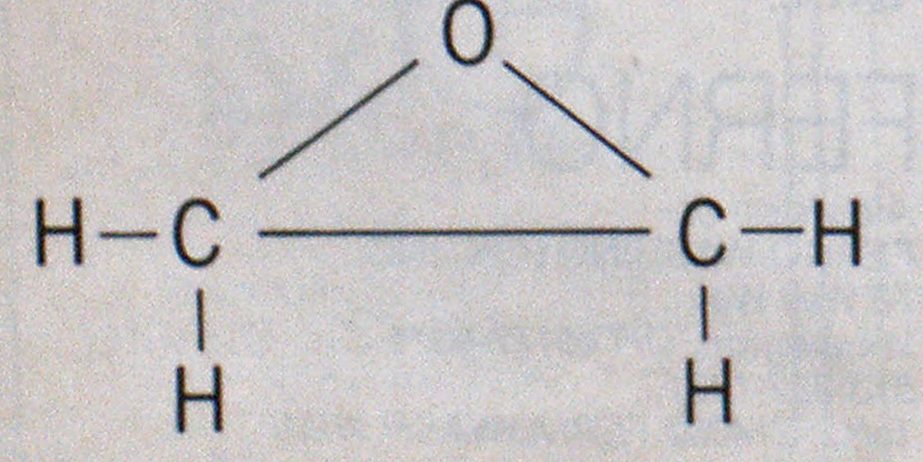
Physically, ethylene oxide is a sweet-smelling, clear liquid, whose odor is somewhat etherlike. It is soluble both in water and in some common solvents (acetone, benzene, alcohols) in all proportions. Ethylene oxide has a specific gravity of 0.88 and freezes at-170.7°F. Its molecular weight is 44, and its molecular structure is unusual for such a small molecule. Its molecular formula is (CH2)2O, and because of its structure, the formula may be found written as CH2CH2O and its molecular structure is cyclical in this nature:

This molecular structure accounts for some of the hazardous properties of the material. The position of oxygen in the molecule and the way the carbons are bonded make the oxygen available to support the combustion of the hydrogen and carbon in the molecule. This means that ethylene oxide may act as its own oxidizing agent, and this is pointed out dramatically by the flammable range of the material.
The ignition temperature of ethylene oxide is 804°F when exposed to oxygen in the atmosphere, but will ignite at 1,060°F with no air present!
But its flammable range is the real danger. Ethylene oxide’s lower flammable limit is 3%, and its upper flammable limit is 100%. This, coupled with the ignition temperature of 1,060°F with no air present, means that the oxygen in the molecule is available to support the combustion of the rest of the molecule, and ethylene oxide may ignite and burn in its own container.
In addition, ethylene oxide, if contaminated with certain materials such as the chlorides of aluminum, iron and tin, iron and aluminum oxides and sodium, potassium and lithium hydroxides, will polymerize instantly, liberating large quantities of heat.
Ethylene oxide has many synonyms, among which are anprolene, benvicide, carboxide, cry-oxide, dihydrooxirene, dimethylene oxide, epoxy ethane, 1,2-epoxy ethane, EO, etheneoxide, ethyloxide, ETO, EtO, NCI-C50088, oxane, alpha, beta-oxidoethane, oxidoethane, oxiran, oxirane, oxirene-dihydro, oxyacyclopropane, oxyfume, oxyfume 12, oxyfume sterilant-20, pennoxide, steroxide-12, steroxide-20, and T-gas.
Its UN/NA designation is 1040, its Standard Transportation Commodity Code (STCC) is 4906610, and its CAS (chemical abstract service) number is 75-21-8. The Department of Transportation (DOT) classifies ethylene oxide as a flammable liquid, and its National Fire Protection Association (NFPA) classification is 2-4-3 and its International Maritime Organization (IMO) designation is flammable gas, 2.1.
USES
Ethylene oxide is primarily used as an intermediate in the production of many other chemicals. The largest volume of ethylene oxide is used in the manufacture of ethylene glycol, a permanent antifreeze, and as an intermediate chemical for polyethylene terephthalate (PET) and polyethylene terephthalate glycol (PETG), two plastics resins used in the manufacture of polyester bottles, fibers, and films.
Home laundry and dishwashing detergents and special surface active agents for industrial use make up the second largest use of ethylene oxide. Next in volume, ethylene oxide is used to manufacture glycol ethers which are used as solvents for surface coatings. The manufacture of ethanolamines used to make soaps, detergents, and textile chemicals consume the fourth largest volume of ethylene oxide, and the rest is used in the production of pesticides, drugs, and many other chemicals. Ethylene oxide is also used as a fungicide, a fumigant, and for the sterilization of surgical instruments.
CONTAINERS
Ethylene oxide is always shipped as a liquid, and it will be shipped in containers as small as glass jars to as large as railroad tankers and all sizes of containers in between.
HAZARDS
Ethylene oxide vapors can irritate the eyes, skin, and respiratory tract, and will leave an unpleasant taste if breathed in large concentrations. It may be detected by smell at concentrations as low as 1 ppm (part per million of air) and it has a TLV-TWA (threshold limit value-time weighted average) of 1 ppm or 2 mg/m3 (milligrams per cubic meter).
Since ethylene oxide is a suspected carcinogen, all contact with skin and eyes, inhalation of the vapors, and ingestion of the liquid should be avoided. There is no STEL (short-term exposure limit) available for this material. Where the liquid may have saturated clothing, skin burns may develop. With all the other hazards of ethylene oxide, the irritating nature of the liquid and vapor may be overlooked.
High concentrations of the vapor may depress the central nervous system through inhalation. Even though there are clearly defined symptoms of overexposure (such as drowsiness, breathing difficulty, nausea, vomiting, and general weakness), all of these symptoms may be bypassed if there are high concentrations of the chemical, which may cause unconsciousness, which could lead to death if the exposure were to continue.
The vapor density of ethylene oxide is 1.52, which means its vapors will stay low to the ground and flow until they find an ignition source. As always with vapors or gases whose vapor densities are greater than 1.0, those vapors or gases may collect in basements or other low, closed-in areas. These concentrations of gases or vapors are extremely hazardous in that they may be in places far from the incident, and may totally surprise inhabitants of the structure.
Ethylene oxide is especially hazardous in these situations because there really is no upper flammable limit for these vapors. Although the flammable range has already been given as from 3% to 100%, the 100% figure really means there is no upper flammable limit. Since ethylene oxide may ignite inside its own container, any mixture of ethylene oxide vapors and air in a basement or other enclosed space can never be too rich, and all that is missing is an ignition source.
Glossary
Aeration—The saturation of a substance with air.
BLEVE—Boiling liquid, expanding vapor explosion.
Easily liquifiable gas—A gas with a relatively high boiling point (-50°F and up).
Polymerization—The chemical reaction in which a special compound, called a monomer, combines with itself to form a long-chain molecule called a polymer.
Runaway polymerization—An uncontrolled reaction with the liberation of tremendous amounts of energy. If the release of the energy is instantaneous, the result is an explosion.
Sparging—The bubbling of air or another gas through a solution.
PERSONAL PROTECTION
These hazards make personal protection absolutely necessary. Whenever ethylene oxide has been exposed to the atmosphere, respiratory protection will be necessary. This means positive-pressure self-contained breathing apparatus (SCBA) should always be used when responding to an incident involving ethylene oxide.
Splash-proof goggles must be worn by workers exposed to ethylene oxide, often under full faceshields. This means emergency responders should have no less protection, although the full faceshield of an SCBA should afford complete protection. The eyes must always be protected.
Impervious protective clothing must be worn by any emergency responder, and this usually means a total encapsulating suit made of butyl or nitrile rubber, or chlorinated polyethylene. There may be other materials that offer suitable protection against ethylene oxide. The manufacturer’s list of chemicals for which its suit offers protection should be consulted. At the very least, anyone coming into contact with the liquid or a high vapor concentration requires eye, skin, and respiratory protection. This may be done with the proper combination of protective clothing other than total encapsulating chemical suits (which might include an umbilical-supplied air supply), but the wearer must be sure that all articles of clothing are impervious, that there is no way for liquid to contact the skin, and that the eyes and respiratory system are protected.
The important thing for the emergency responder to remember is that he must protect himself from both liquid and high vapor concentration contact that might make the total encapsulating suit mandatory.
HANDLING
Fire situations
Response to a fire involving ethylene oxide is every bit as tricky and dangerous as responding to a spill. As always, if a spill is involved and no fire is present, you must do what you have been trained to do in all accidental releases of a highly flammable liquid:
- Approach from upwind;
- Eliminate all ignition sources;
- Try to disperse the flammable vapors;
- Consider evacuation for a safe distance in all directions, at least ten times that distance downwind;
- Keep all but necessary emergency personnel out of the danger area and try to keep the liquid from spreading (the larger the surface area of the spill, the more rapid the production of vapors).
If a fire does exist, even though ethylene oxide is classified as a flammable liquid, you should treat it as an easily liquifiable gas. This means that you should not extinguish fires of the leaking product until the flow of the liquid (or the escaping vapor) can be stopped.
The potential for a BLEVE (boiling liquid, expanding vapor explosion) of a container of ethylene oxide is high. The possibility of a runaway polymerization explosion is also present. Whether the explosion is a runaway polymerization type or a true BLEVE is immaterial, however, since the potential for loss of life and property damage is essentially the same.
Emergency procedures in case of a fire include the attempt to cool the containers upon which flames or radiant heat are impinging. It is very important to remember that exposure to radiant heat may cause a container to rupture just as surely as impinging flame. This may cause more deaths and damage simply because emergency personnel want to attack the flames and cool those containers in or near the flames, often positioning themselves between the flames and other containers of pressurized gases and flammable or combustible liquids.
Remember that the boiling point of ethylene oxide is so low that in a fire it might react like an easily liquifiable gas, presenting additional hazards to those already provided by the flammable liquid. The action called for here is the cooling of all containers that may be subjected to excessive heat. Certainly the containers in the greatest danger of catastrophic failure are those being impinged by flame; just don’t overlook those being subjected to the radiant heat of the flames, and never position yourself so that if failure does occur, you will be trapped by flames from behind. The principal here is to look for all containers that are being stressed, not just the obvious ones, and stress in this case means being subjected to heat.
A word of caution should be injected here. If ethylene oxide is to be treated like an easily liquifiable gas, one of the rules in fighting a fire in a material like that is never to extinguish the flames until the flow of fuel may be stopped. However, ethylene oxide, you will recall, is subject to runaway polymerization. This can be produced by the radiant heat emanating from the flame of the escaping vapor.
In case of a spill that causes a pool of burning liquid to form, water may be an ineffective extinguishing agent. If water is used, it should be as a fog or spray, attempting to cool down the liquid below its ignition point. Alcohol foam will be effective, and should be the extinguisher of choice. Dry chemical will be effective on smaller fires, and CO2 will extinguish an ethylene oxide fire if it is in an area protected from the wind. Be advised that with such a low boiling point, the addition of water may actually warm the material and cause it to evaporate faster.
Containment
Where the material has been spilled and no fire is present, care must be taken to prevent it from spreading. Soil and sand may be used as diking materials, and pits may be dug to contain the liquid.
Since ethylene oxide evaporates so rapidly, containing the liquid behind dikes will prevent its flowing, but vapors will be constantly produced. Hose lines may be positioned downwind to help disperse the released vapors, which may also be dissolved in the water spray or fog application.
Whenever containment techniques are used, ethylene oxide may seep into the soil, contaminating it. Such soil may be required to be removed after an incident.
If a pit is dug, a cover of some sort (tarpaulin, plastic sheeting) may be used to cover the pit to restrict the production of vapors. If containment behind a dike is effected, alcohol foam may be applied to the surface of the liquid to restrict the generation of vapors. The foam may break down in a short while and may be reapplied. Be aware that every application of foam adds to the volume of liquid contained, and repeated applications of foam may cause an overflowing of the dikes.
All manholes must be blocked, as well as any entrances to waterways. If spilled ethylene oxide enters a waterway, the proper environmental authorities and all users downstream must be notified of the contamination.
Alternative actions
After containment, dilution may be used to eventually slow the production of vapors. However, just the opposite will occur at the beginning of the addition of water, with production of vapors being particularly bad if the water is warmer than the ethylene oxide. Eventually though, the addition of water will significantly raise the flash point of the solution as vapor evolution is decreased.
Remember that whenever water is used in an ethylene oxide release, whether as a spray or fog to dissolve or disperse vapors, as an extinguishing agent, as a diluent, or as a component of foam, you must be aware of the increased volume of liquid and keep control of the runoff.
Another alternative method of handling spilled product that is contained in a dike or pit is by pumping it into secure containers. If portable pumps are to be used, make sure that explosion-proof switches and motors are used. As always, work upwind, prevent the accumulation of vapors, and eliminate all ignition sources.
Where ethylene oxide has entered a waterway and all downstream users have been alerted, efforts can be made to decontaminate the water. These techniques include some of the same techniques used by water-treatment plants in purifying water. Agitation, aeration, and sparging may be used to remove the contaminant. However, remember that ethylene oxide is very flammable, and whenever its vapors are released, a fire is possible.
The incident commander is now faced with a serious dilemma. Does he order the flames extinguished and face a possible explosion from the concentration of unburned vapors that will now collect? Or does he keep trying to cool the containers and protect exposures while waiting for a BLEVE-like explosion?
The answer, of course, will always be dictated by the situation on the fireground, but it would seem to make the most sense to continue to try to cool the containers and protect exposures, all from unmanned monitors or from “explosion-protected” positions. If the explosion does occur, at least no one will be injured.
FIRST AID
Where a victim has been exposed to, and probably inhaled large quantities of ethylene oxide, he must be removed to fresh air immediately and kept warm and quiet. Artificial respiration should be used if breathing becomes labored or ceases, and always keep in mind that the person administering the mouth-to-mouth resusitation will be exposed to the vapors.
If the chemical has been ingested, the victim must drink large quantities of water and be induced to vomit, while conscious, of course. He should have medical attention immediately.
Where a victim has had skin and eye contact with ethylene oxide, the affected area should be flushed with water for at least 15 minutes. All contaminated clothing must be removed immediately.
SUMMARY
Ethylene oxide is a flammable liquid that produces tremendous quantities of explosive vapors. Its extremely wide flammable range means that the vapors of ethylene oxide (which will hug the ground or accumulate in basements or other low-lying areas) can never be in a “too rich” condition, and explosions are likely with any common ignition source (even inside its own container). It will be found in many industrial settings and in every transportation mode.
Since ethylene oxide can cause severe irritation on contact and is a suspected carcinogen, handling in an emergency situation calls for great caution and complete protection.

