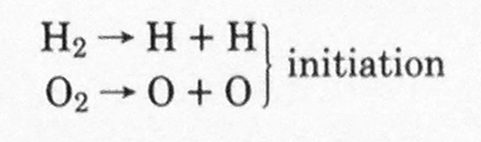
Chemical Reactions Sustaining Fire: How Oxygen Affects Combustibles
features
Once a fire spreads beyond the incipient stage, it becomes increasingly difficult to extinguish. Why this is so lies in the nature of the fire process and the means employed to deal with it.
Fire is a chemical reaction between oxygen and a burnable material with the evolution of heat, light and smoke. This is a simple explanation of fire and were this all there is to the act of burning, then extinguishment would be simple, too. We know, however, that extensive fires are exceedingly hard to bring under control.
Fire reaction, like all chemical reactions, is increased in speed and intensity by such factors as temperature, state of subdivision, concentration of the reactants and presence of catalysts.
Effect of catalysts
Catalysts increase the speed of reactions by their presence and suffer no net change after the process is over. Catalysts can exert their powers at the very beginning of the reaction, or they can affect the rate as they are produced during the burning action itself. When formed during the combustion reaction, they are called autocatalysts.
These byproducts of the fire reaction exert a tremendous influence on the rate of burning, increase the ferocity of conflagrations and present tremendous problems in extinguishment.
To better understand this, let us consider the least complicated example of burning, that of hydrogen and oxygen, to produce water:
Energy in the form of heat and light is also produced.
The equation shows the net reaction as though it occurs in just one step. The combustion, however, does not proceed entirely as shown. It moves, instead, through a number of steps, which considered together may be described as a reaction pathway. During some of these steps, certain short-lived, highly reactive substances are formed that accelerate the rate of reaction. Furthermore, these fragmentary species are continually being regenerated in a process called a chain reaction.
2H2 + 02 – 2H20
The whole process proceeds in three stages. The first stage is called chain initiation, in which heat energy acts on the hydrogen and oxygen molecules, breaks the normal chemical bonds, and produces fragments, each with an unpaired electron called free radicals.
Electrons normally spin in atoms and molecules and act as small magnets. They must be paired so their effects are balanced. When an atom or molecule has one unpaired electron, it becomes highly energized and strives to combine with another atom or molecule that also has an unpaired electron but of an opposite spin. When this happens, a great amount of energy is released and transferred to the reacting system.
In the initiation steps, partial dissociation of hydrogen and oxygen molecules takes place, producing H and O free radicals:
Hydrogen and oxygen molecules may also react. Then OH free radicals are produced:
H2 + O2 → OH + OH – initiation
The free radicals, O, H and OH, then combine with more oxygen and hydrogen molecules to produce additional free radicals. In this way, these transitory substances become propagators, or carriers, of the chain and react over and over again until all reacting substances are used up:
The propagation steps take place in a fraction of a second and are the keys to the extremely fast burning reaction, with the OH free radicals playing a most important role.
Special chain propagation
A special type of chain propagation takes place when a hydrogen free radical attacks an oxygen molecule, as in the first step above. In this case, for each hydrogen free radical destroyed, two more free radicals are produced, OH and O. Then each of the two new free radicals in turn reacts with more molecules to form two more free radicals and so on.


The reaction of oxygen free radicals with hydrogen molecules also produces two free radicals for each oxygen free radical used up, as shown in the second step above. These special combinations are called branching chain reactions and are characteristic of all explosions. In fact, it is a branching chain reaction that is responsible for nuclear explosions, as well as the explosions of gasoline in the cylinders of internal combustion engines that lead to knocking.
The chain reactions need not necessarily go on until the reacting substances are entirely consumed. Certain influences can bring the reactions to a halt in a series of steps called termination. The presence of large surface areas or substances that combine with or otherwise trap the chain carriers will stop the reactions. Free radicals can also combine with each other to form relatively inactive species, such as water, oxygen molecules or hydrogen molecules, which will slow down and finally stop the process.
Many more steps have been identified in the hydrogen-oxygen reaction than these, all of which add to the complexity of the whole action.
Complicated process
If from all of this, we conclude that such a simple combustion reaction as that between hydrogen and oxygen is actually very complicated, then consider the absolute futility of comprehending the mechanisms taking place when already complex materials such as wood, fuels, plastics and other common combustibles are subjected to the burning process.
In fire, these materials break down pyrolytically to a myriad of flammable substances, each of which goes through the steps of a chain reaction in the flames. Imagine trying to fathom the pathway and reactions involved as wood burns. At least 100 organic products have been identified in the flames of wood fires. Each of these substances goes through the process of a chain reaction that may involve as many as a dozen or more individual steps. This very involved interaction of many energetic reactions presents a difficult problem for fire fighters trying to control such an inferno unless these very mechanisms are used to bring about extinguishment through a chemical action.
But our customary extinguishing methods rely heavily on the use of water to exert a basically cooling effect on the burning material, and this is relatively ineffective against the chain reactions going on in the flames.
Inhibiting possible
It is these free radical chain reactions, in which a small number of energetic intermediaries can produce many more active species, which are the keys to the extremely violent burning action. It follows, then, that the removal or destruction of these fragments should result in a dramatic halt in the burning rate. This is not only possible, but it is a process already available to us in the presently limited use for extinguishment purposes.
The most spectacular use of this chemical inhibiting process to control violent reactions is the use of graphite rods in nuclear plants to moderate the rate in atomic reactions so that the otherwise awesome explosions can be used for generating electrical energy.
In our automobiles, lead tetraethyl is used to prevent the harmful knocking explosions as the gasoline burns in the cylinders.
In the fire service, dry chemical extinguishing agents, such as sodium and potassium bicarbonate, are being used to exert strong chemical effects in extinguishing fires. These chemicals break down in the flames to produce substances that react with the OH free radicals and inhibit their action. In addition, the finely divided powder particles provide an enormous surface area per unit mass to form a heat barrier and also trap other free radicals by a process called adsorption. The adsorbed species combine and liberate a great amount of heat energy which is dissipated on the huge surface area. Extinguishants of this type are Purple K, ABC, Super K and Monnex.
Halogens as suppressors
Compounds of substances called halogens, such as fluorine, chlorine and bromine, also act as powerful suppressors of the free radical chain reactions in flames, and extinguishing agents like Halon 1301 are being used as effective control agents even in low concentrations.
Halogens have the added advantage of chemically combining with the hot char and altering the pryolysis process, thereby reducing the amount of flammable gases produced. This aids control because the flame itself is deprived of a continuous supply of fuel. By this process, too, flashback is reduced.
The older extinguishants, carbon tetrachloride, bromine hydrocarbons, and loaded stream mixtures, are known to exert chemical effects on flames and usually control a fire with small applications.
Chemical inhibitors not only exert their effects through a combination of free radical trapping, chain breaking and surface active processes, but they also proceed through chain reactions of their own. These chain reactions, however, are just the reverse of the chain reactions which contribute to the burning process. Thus, a little of the inhibitor affects a large amount of the burning species. This is why chemical inhibition promises to be so efficient as a fire control measure.
Other actions occurring
Returning to the discussion of the burning process, there are other actions occurring which tend to increase the intensity of burning.
As an example, atoms and molecules subjected to high temperatures have some electrons stripped from their structures and become ionized. This is a state in which the atoms or molecules carry a positive electric charge and become extremely reactive. All substances that burn exhibit this property of ionizing and the more complicated the molecule, the higher the degree of ionization. The flames of organic substances, such as acetylene, gasoline or wood, shown an ionization potential about 10 times higher than hydrogen flames.
Ionized particles differ from free radicals in that ionization produces an electrically unbalanced fragment, while in free radicals the effect is due to unpaired magnetic spinning of the electrons, with the fragment itself being electrically neutral.
Use of electric fields
Here again, the capacity exists for an effective means of extinguishment using knowledge of the chemical ractions involved. Studies have shown that electric fields can be used to alter flames and aid in final suppression. Explosion waves have been subdued by directing the ionized flame-front toward a negative pole in an electric field. While this seems to be impractical for use in dayto-day fire fighting, the process may have application in stationary on-thespot protective systems for specific hazards. Or additives might be found that will convert water or other liquids into a properly charged agent to alter the reactive charges in flames to stop the burning.
New branches of science have recently emerged in which lasers have been used to affect chemical bonds in the course of chemical reactions. Further research may indicate a means to control the combustion reactions by laser perturbation of the chain and ionization processes in flames.
Or perhaps a close examination of the human metabolism process may point the way to efficient and safe extinguishment. One must consider the marvelous processes going on in our bodies that permit a variety of combustible food processes going on in our bodies that permit a variety of combustible food fuels to be oxidized in the cells to provide heat and energy with the production of carbon dioxide and water. These reactions are controlled by catalytic inhibitors called enzymes and conduct the processes at an amazingly constant temperature of about 37° C (98.6°F). This process has not been possible to duplicate in the laboratory.
Insight increasing
Yet each day we are gaining remarkable insight into the mechanisms that make and break fire reactions, and some extinguishing methods already appear to be the precursors of the real means of combating severe fires effectively. Somewhere in the mechanisms discussed lie the answers leading to the ideal extinguishing process. The search for this must be relentless and must occupy the serious efforts of the present and future generation of fire fighters.
Traditional methods of combating fire using water as the main extinguishing agent has been, and will continue to be, less than perfect. The fire service must direct its resources to developing alternate means to control fiery reactions just as the atomic scientists have been forced to develop means to turn the terrifying force of nuclear explosions into controlled reactions for the use and benefit of all mankind.
When we uncover the solution to these problems, we will have found the perfect way to control conflagrations. That day should not be too far into the future, providing the necessary research is carried forward to this end.

