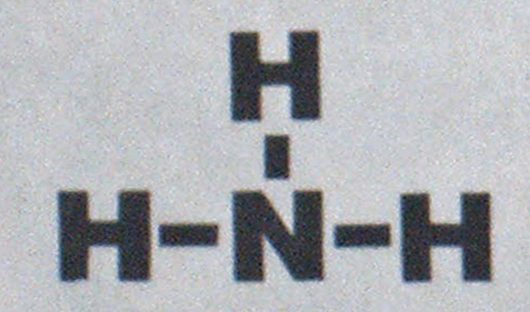
Ammonia
FEATURES
HAZARDOUS MATERIALS
Chemical Data Notebook Series:
Ammonia, many times referred to as anhydrous ammonia, is a colorless gas with a bitter taste and a sharp, intensely irritating, characteristic odor. It is one of the highest volume chemicals produced in the United States, and is found in industry, agriculture, and in all modes of transportation, including so-called off-the-road (farming).
PROPERTIES
Ammonia is a gas in its natural state, and because of its relatively high boiling point (-28°F), is easily liquifiable. Since so much more material can occupy the same space in a liquid form rather than as a gas, ammonia is usually shipped as a liquid. It is colorless as a liquid, but as it vaporizes it may take on a white appearance as it condenses water out of the air.
Ammonia is very soluble in water, forming an alkaline solution of ammonium hydroxide. Its specific gravity as a liquid is 0.68, and its vapor density as a gas is 0.59. It freezes at -108°F.
A stable compound, ammonia’s molecular formula is NH„ its molecular weight is 17.0, and its structural formula is:

Since ammonia is easily liquifiable, it has found use as a refrigerant.
IDENTIFICATION
The UN/NA designation for ammonia is 1005, its STCC (Standard Transportation Commodity Code) is 4904210 as anhydrous ammonia and 4904220 as a solution of more than 44% ammonia in water. Its U.S. Department of Transportation (DOT) classification is non-flammable gas, its IMO (International Maritime Organization) classification is 2.3, poison gas, the NFPA (National Fire Protection Association) 704 rating for ammonia is 2-10 as a gas and 3-1-0 as a liquid. Ammonia’s NIOSH (National Institute for Occupational Safety and Health) or RTECS (Registry of Toxic Effects of Chemical Substances) number is BO 0875000, and its CAS (Chemical Abstract Service) number is 7664-41-7. Its shipping name under IMO is ammonia, while DOT calls it ammonia, anhydrous.
Ammonia is known by several synonyms, including AM-fol, ammonia gas, ammonia anhydrous, liquid ammonia, Nitro-sil, and spirit of hartshorn. Aqua ammonia is a solution of ammonia in water, also known as ammonium hydroxide, ammonia solution, and ammonium hydrate. Technically, ammonium hydroxide is a corrosive liquid containing not more than 30% ammonia dissolved in water.
HAZARDS
Anydrous ammonia is classified by DOT as a non-flammable gas because its lower flammable limit is 16% (the minimum lower flammable limit to be classified as a flammable gas is 14%), and its flammable range is 16% to 25% (the minimum flammable range for a gas to be classified as flammable is 10%). However, ammonia will burn, as soon as a concentration of 16% is reached and its ignition temperature of 1,204°F is reached. Burning may not be the greatest hazard of ammonia, but its classification as a non-flammable gas can lead emergency responders into the dangerous assumption that it will not burn.
The greatest hazard of ammonia may be its toxicity. It can be detected in a concentration as low as 0.04 ppm (parts per million) and has a short-term exposure limit (STEL) of 35 ppm (the time limit for STELs is usually 15 minutes). Exposure to 5,000 ppm will be fatal almost immediately.
One will not voluntarily stay in an atmosphere containing ammonia in any appreciable quantity. The smell is very sharp and pungent, and the effect is very irritating; much coughing and choking will take place. The sharp feeling and taste is a result of the ammonia gas dissolving in the moisture of the nose and mouth, forming ammonium hydroxide in the following manner:
NH3 + HOH — NH4OH
(Ammonia plus water
yields ammonium hydroxide)
The reverse of this reaction will occur if heat is applied to ammonium hydroxide, yielding water and ammonia.
Ammonium hydroxide is known as a base, and is very alkaline or caustic, causing severe burns to the skin when high concentrations are in contact. As a vapor, 700 ppm to 1,000 ppm may cause serious eye damage all the way to blindness. At levels below these, the eyes will be tearing and smarting.
Contact with liquified ammonia will cause frostbite due to its low temperature (-28°F), and tremendous quantities of gas will be generated as the liquid boils away. Needless to say, a splash that reaches the eyes could have catastrophic effects. Another hazard exists in the manner in which the material is shipped. As a liquid or as a gas under pressure, the containers will be subject to tremendous stresses if heat causes the temperature of the product to rise. A container holding the liquid is usually insulated, but impinging flame or prolonged radiant heat can eventually cause pressures within the container to rise. A failure of the container can result in flying metal and rapidly moving product. Whether or not a BLEVE (boiling liquid, expanding vapor explosion) is possible is a matter for experimentation and study.
REACTIVITY
Ammonia reacts dangerously with many materials. It evolves heat as it dissolves in water. Ammonia reacts with acids, aldehydes, amides, isocyanate, organic anhydrides, strong oxidizers, and several metals. It is corrosive to aluminum, copper, lead, tin, zinc, and many alloys. Ammonia may form explosive compounds with calcium hypochlorite, gold, mercury, and silver.
USES
Ammonia is used in many aspects of American business, being found in industry, agriculture, and in many commercial uses. It is a very popular and efficient fertilizer (its formula NH, shows it is 82.3% nitrogen by weight). Ammonia is so efficient, in fact, that it may be injected directly into the soil as a liquid.
It has already been mentioned that because of ammonia’s relatively high boiling point, it qualifies as a good refrigerant. Because of this, ammonia may be found in many unexpected commercial establishments. Ammonia was once used extensively as a refrigerant in many large walk-in coolers. Therefore, fires in old sections of town, where corner grocery stores were once prevalent, may produce ammonia in substantial (and combustible) quantities. Industry uses tremendous amounts of ammonia in the manufacture of cleaners, dyes, explosives, fibers, pharmaceuticals, plastics, and many other chemicals and processes.
PROTECTIVE CLOTHING
Emergency responders must protect themselves from contact with ammonia in both the liquid and gaseous form. Full protective gear will be effective as long as it includes gloves, boots, and other clothing that is impervious to ammonia and that may be worn in a manner to prevent the gas or liquid from passing through seams, sleeves, and other openings, plus positive pressure self-contained breathing apparatus (SCBA).
Fully encapsulating suits should be worn whenever there is a chance of high concentrations of the vapor being present in the air. Manufacturers of suits claim that natural rubber, butyl rubber, nitrile rubber, neoprene, and polyvinyl chloride (PVC) are compatible materials. Remember that the thickness of the encapsulating material and the length of contact with the product have a lot to do with the level of protection offered by each material. The manufacturers of your suits should be contacted for verification of protection, and tests should be run on a sample of the material in advance of any use.
CONTAINERS
Ammonia is transported and stored in various sized containers, ranging from familiar cylinders holding the gaseous product, to rail tank cars holding 30,000 gallons of liquified gas and stationary containers holding even larger quantities. River barges and pipelines are also common methods used to transport ammonia. Intermediate size containers include small tank wagons and those tanks used by farmers to apply ammonia directly into the soil.
All containers will contain product under pressure, and as such will be subject to catastrophic failure if subjected to intense heat over a prolonged time.
HANDLING
Fire situations
It must be mentioned again that although ammonia is classified by DOT as a non-flammable gas and by IMO as a poisonous gas, ammonia does and will burn. While it may be difficult to get as much as 16% concentration of ammonia in air (and in a massive spill, less than 25% in air), its ignition temperature of 1,204°F is not difficult to reach. While it is certainly true that a 16% concentration of any gas in air is very high and unlikely (excluding, of course, oxygen and nitrogen), the lower limit is easily reached near an accidental release of the liquified gas.
The important thing to remember is that hazardous material response teams and other emergency responders don’t operate under Murphy’s law (If anything can go wrong, it will), but under O’Brien’s Law (Murphy was an optimist). The instant that an emergency responder decides that he is safe from fire because ammonia is a non-flammable gas, he will find himself within that flammable range, and may even provide the ignition source himself.
Glossary
Aeration—A method of purification of liquids; spraying the liquid into the air or bubbling air through the liquid.
Alloy—A physical, intimate mixture rather than a chemical change.
Anhydride—A chemical with the water removed.
Anhydrous—No water present.
Bolling point—The temperature at which the vapor pressure of a liquid just equals atmospheric pressure.
Easily liqulfiable—Pertains to a gas with a relatively high boiling point. Gases may be liquified by either applying sufficient pressure, or cooling the gas to its boiling point (a combination of pressure and cooling will liquify the gas above its boiling point).
Flammable range—The proportion of gas or vapor in air between its lower and upper flammable limits.
Lower flammable limit—The minimum concentration of gas or vapor in air below which it is not possible to ignite the gas or vapor.
Upper flammable limit—The maximum concentration of gas or vapor in air above which it is not possible to ignite the gas or vapor.
Ammonia should be treated as any other easily liquifiable gas that burns. That is, the product is stored in an environment that is substantially above its boiling point, and in the case of impinging flame or other intense heat source, may be subject to a BLEVE. In this case, care must be taken in making the decision to attack. The container must be kept cool, but the nearness of firefighters, holding handlines or operating deluge guns may place them in danger if the container explodes.
Approach should not be attempted if the container has been subjected to intense heat for a prolonged period of time, especially if the heat source is directly impinging flame, and the impingement is on the vapor space above the liquid. This sets up the classic BLEVE situation: the rising pressure in the container attacks the area of the tank weakened by the metal fatigue that was caused by the flame impingement. When the weakened area fails, the container usually opens up longitudinally (if it is a cylindrical tank), releasing a tremendous amount of vapor (and liquid in a mist form). Even though in the center of this release, the concentration of ammonia in air is 100%, somewhere at the outer edge of the vapor the flammable range of 16% to 25% will exist, and the flames of the fire will ignite it. The fireball may not be as impressive as if it were propane involved, but dead emergency responders are hard to impress anyway.
If the container can be reached by water streams, massive amounts of water must be applied to cool the container in an effort to prevent a BLEVE. The safest application is with the use of unmanned monitors. Evacuation should be effected fora minimum of one-half mile for large tanks, and a minimum of one-fourth mile if a cylinder is involved. Withdrawal of emergency responders should also be to these distances.
Where pools of liquid are burning, water fog or spray may be effective in cooling down the fire, and the application of certain types of foam may also be effective. For very small pools, CO2 (carbon dioxide) or dry chemical may be effective, depending on wind conditions.
It must be remembered that liquid ammonia is at least -28°F, and the application of water (which is at least 60°F warmer and sometimes 70°F to 90°F higher than the temperature of the chemical) will accelerate the production of gaseous ammonia. Therefore, the first application of water, if there is no fire, may cause the lower flammable limit of 16% to be reached quickly. If the pool is burning, application of a stream of water may splash the burning product around.
Accidental release
Where gaseous ammonia is accidently released, fire or toxicity problems may not arise due to the vapor density of the product. At a vapor density of 0.59, the gaseous product will quickly rise and disperse in air. The leaking material will be forced out by the internal pressure within the container, and if the gas is being forced upward, the danger is lessened. As the gas escapes, it will be cold, and this may increase its vapor density somewhat. This means that some product may be near the ground for a short time, so one cannot always count on rising gaseous ammonia immediately after a release.
Gas released by a pool of liquid ammonia will also rise and disperse, but slowly at first due to its low temperature. The rate of gas evolution depends on the amount of liquid spilled, the area over which it has spread (the larger the surface area of the spill, the more gas will be released), the temperature of the ground and air, and the weather and wind conditions.
Evacuation downwind must be considered, even though the gas will begin to rise as it moves away from the spill. Again, one must consider the humidity, and/or the possibility of rain. The solubility of ammonia in water is so high that ammonium hydroxide will be formed. This could negate the evaporation and quick dispersal of the gas, and it may be held close to the ground by the moisture in which it is dissolved. This will heighten the danger to inhabitants near the spill and may hasten evacuation orders. It also means that the vapors may be “swept” from the air by use of a spray or fog. Just be sure to remember to contain the runoff.
Containment
Containment is a technique that must be attempted, even though the liquid may begin evaporating very rapidly. A containment pit may be a better strategy than building a containment pond by diking around the spill with soil, sand, or clay, simply because the surface area of the liquid contained in a pit will probably be smaller than in a pond, and hence the slower production of gases.
Also, a containment pit is easier to cover with a tarp or plastic sheeting of some sort which will cut down the evaporation rate. Once contained, liquid ammonia may also be covered by a foam to cut down evaporation rate. The proper foam must be selected, and it must be remembered that any method to reduce production of vapors may also concentrate them or prolong their porduction over a considerable period of time.
If containment dikes or pits have been used, considerable soil contamination may have occurred. Clean up crews (professional) will have to remove all soil that has been contaminated.
Remember that the gas may be removed from the air by sweeping motions of a spray or fog nozzle. Also remember that the ammonia dissolved in water is now a basic substance, ammonium hydroxide, and the runoff must be controlled.
The liquid ammonia must also be prevented from entering sewers, as highly dangerous concentrations of ammonia gas will quickly build up in the system. This may be helpful in controlling the sewers’ rat population, but the gas will also be harmful to humans. (Of course, the gas may cause another problem by driving rats out of the sewers and onto the streets before it s able to kill them.)
Dilution
Dilution is a technique that can usually be used only on smaller spills. Remember that as you add water to effect the dilution, the relatively warm water will cause a speedup in the production of ammonia gas until the solution is very dilute. This solution will have to be removed and disposed of properly.
Removal
The best action may be to remove the spilled product by suction into approved containers. The shipper may provide another truck and vacuum equipment to capture the spilled product from its containment pond or pit, and/or off-load the leaking vehicle. The shipper may use two trucks, as he may not want to mix contaminated (spilled) liquid with uncontaminated material still in its original container.
Neutralization
After the ammonia has been dissolved in water and formed the basic ammonium hydroxide, weak acids may be used to convert the ammonium hydroxide to a harmless salt. The producers of ammonia should be consulted to determine what a suitable neutralizing agent might be.
If product does enter a waterway that cannot be diverted and/or contained in some manner, all downstream users of the water must be notified of the contamination as should the proper federal, state, and local environmental authorities.
Sewage treatment plants must be notified, for obvious reasons, and may be helpful in removing the dissolved ammonia from the water passing through the system. Sewage plants usually use a technique called aeration as one of the water purification steps. This spraying of water allows contact of small particles of water by the oxygen in air, which speeds up the purification by oxidation. This same method will drive the ammonia from its water solution (which will, of course, liberate free ammonia gas). However, under controlled conditions, it may be a safe procedure.
The sewage plant might also use a technique called sparging, in which bubbles of air are forced through a liquid (and sometimes solids and gases). This bubbling action will also encourage the ammonia to leave the solution.
SUMMARY
In summary, ammonia gas has many hazards, among them are its toxicity, flammability (notwithstanding DOT classification), reactivity, corrosiveness, and ability to cause frostbite in its liquified form. Ammonia is very hazardous in its pressurized form, as is any pressurized gas.
Ammonia is found in industry and agriculture, and in a surprise form as a refrigerant in some old walk-in coolers in old grocery stores. Like any other hazardous material, ammonia can be handled safely with the proper equipment, training, and techniques.

