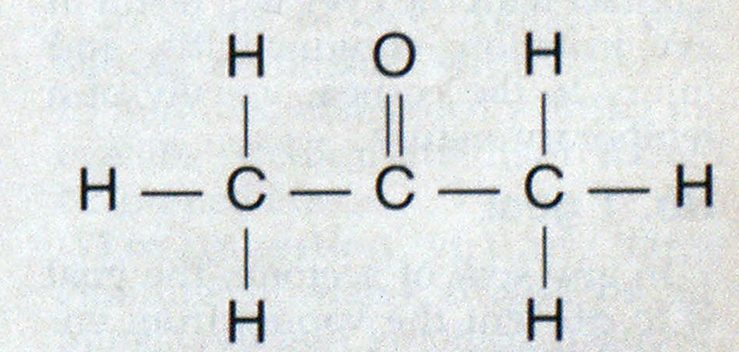
Acetone
HAZARDOUS MATERIALS
Chemical Data Notebook Series:
The first priority of emergency crews responding to an acetone spill must be to prevent an explosion.
Acetone’s flammable range is 2.1% to 13% in air, which means it doesn’t take much released material to quickly produce enough vapors to exceed the lower flammable limit. Being volatile, acetone will produce those vapors rapidly and at a low temperature. Its flash point of 0° F means it will produce vapors sufficient to form an ignitable mixture with the air near the surface of the liquid or its container at 0° F or above. And with a vapor density of 2.0, acetone’s vapors will “hang” together near the ground and flow along any low spots, accumulating in any confined space near or below the ground.
Acetone is a flammable, colorless liquid with a sweet, fruity odor that may be recognized as the odor of nail polish remover. Very sensitive individuals can detect acetone’s odor when it’s present in concentrations as low as 1 part per million of air; others may not notice it at less than 300 ppm.
The chemical’s major use is as a solvent for paints, lacquers, varnishes, cellulose acetate, plastics, and other organic materials (including nail polish). It’s also used in metal cleaning and in industries that produce adhesives, pharmaceuticals, pesticides, and lubricating oils, among other things.
There are several other names for acetone: beta-ketopropane, dimethyl formaldehyde pyroacetic ether, dimethyl-ketal, dimethyl ketone, ketone propane, methyl ketone, propanone, 2-propanone, pyroacetic acid, and pyroacetic ether. It’s usually shipped, however, as acetone.
The U.S. Department of Transportation classifies it as a flammable liquid, and the IMO (International Maritime Organization) classifies it a flammable liquid, 3.1. Acetone carries the UN/NA (United Nations/North America) designation 1090, the STCC (Standard Transportation Commodity Code) number 4908105, the CAS (Chemical Abstract Services) number 6764-1, and the RTECS (Registry of Toxic Effects of Chemical Substances) number AL3150000. The U.S. Coast Guard’s CHRIS (Chemical Hazard Response Information System) code is ACT.
Acetone’s chemical formula is CH3COCH3, and its structural formula is It has a molecular weight of 58.

The autoignition temperature for acetone is 869° F, which is low enough to be reached by all common ignition sources. It boils at the relatively low temperature of 133.2° F, and freezes at -137.7° F.
The liquid dissolves in water in all proportions, but because it has a specific gravity of 0.79, it will float on the surface until it does so.
Acetone isn’t water-reactive, but it is somewhat toxic. The threshold limit value (TLV) for acetone is 750 ppm, and the short-term exposure limit (STEL) is 1,000 ppm for 15 minutes.
Immediate effects of breathing the fumes may be narcotic and anesthetic in nature, so care must be used to protect oneself from exposure to the fumes. The anesthetic and narcotic actions occur as the concentration of vapors reaches 12,000 ppm (1.2% in air), which is still below the lower flammable limit. The victim will experience headaches, dizziness, confusion, nausea, drowsiness, and unconsciousness. Repeated exposure above the TLV can cause liver and kidney damage. Needless to say, if the concentration becomes high enough to reduce the oxygen content to a dangerously low level, death can occur by asphyxiation.
Health hazards of breathing lower concentrations aren’t serious, except to those who might have an allergic or asthmatic reaction. Not only can constriction of breathing passages occur, but pulmonary’ edema can occur in serious cases.
When ingested, acetone will cause irritation of the mucous membranes, nausea, vomiting, and gastritis. And because of its solvent nature, acetone in contact with the skin may cause defatting. This dries and cracks the skin, sensitizing it to other chemicals. Contact with the eyes will result in eye irritation, conjunctivitis, and injury to the cornea, usually of a temporary nature.
No. 1 goal
In any spill of acetone, the goal is to prevent the vapors from entering the flammable range. Simultaneously, you must eliminate all ignition sources. This, naturally, means approaching from upwind and conscientiously working downwind to evacuate while eliminating sources of ignition.
Alcohol-type foam will restrict the production of vapors as long as the surface of the foam retains its integrity. This is a problem for ordinary alcohol foams, because the acetone tends to cause a fairly rapid breakdown. There are specialty foams on the market that may indeed solve this problem. Tests and trials are the only way to determine the actual performance; manufacturers will be happy to arrange such demonstrations.
A water spray or fog over the spill area and immediately downwind will cause some of the vapors to dissolve in the water and help disperse others. Anytime water is used, though, the runoff must be contained.
Although foam application is the first step considered in an acetone spill, containment will be effective if done properly. In creating containment ponds, dikes, or pits, the work must always be performed from upwind; weather and terrain conditions might make that difficult. Other considerations, such as location of the spill in a business section or heavily populated area, may rule out containment.
However, if it’s possible to do safely, digging a containment pit or building a pond can be the best approach. If the acetone can be surrounded in a relatively small area or caused to flow into a deep pit with a small surface area, it will be easier to control the production of vapors. It’s much easier to cover an area of 100 square feet w’ith a tarp or foam than it is to cover a 1,000or 10,000-square-foot area. Even if covering or blanketing isn’t possible, the generation of vapors will be slower from a smaller surface area.
Using containment procedures may reduce contamination of the surrounding land, but it might also allow increased percolation of the acetone into the ground under the pit or pond, threatening groundwater supplies. If there’s no danger to life during mitigation, emergency responders must be alert to their responsibility to protect the environment.
Glossary
Absorption—The penetration of one substance into the inner structure of another.
Adsorption—The attraction of one substance to another, which holds the first on the surface of the second.
Defatting—Dissolution of the fat from human skin by a solvent.
Sparging—A process by which air or another gas is bubbled through a material.
Volatile—Capable of vaporizing readily, a characteristic that’s indirectly related to a product’s flash point.
In containment, as in other techniques, care must be taken to use only spark-proof tools and explosion-proof equipment. This is also important if you have successfully contained the material and are now going to pump it into a secure container. Pumps and hoses must be grounded and bonded to prevent static discharge.
Dilution
Since acetone is soluble in water in all proportions, it’s possible to create a solution that’s no longer flammable. Enough water can be added to spread out the acetone molecules in the solution so the flash point will rise above the temperature of the liquid. In other words, as the concentration of acetone in the solution drops, the solution’s flash point rises. The addition of water also cools the acetone, so that at the point at which the temperature of the solution falls below the flash point, the generation of combustible vapors will cease, and the fire will go out because of insufficient fuel.
The problem with dilution is what to do with the runoff. The resulting solution will still contain acetone and must be handled. There are procedures to remove or recover the product, but this should be done only by clean-up professionals.
Yet another option for controlling an acetone spill is the use of absorbents such as soil, sand, clay, absorbent powders, or one of several commercial sorbents. The problem is that the sorbent materials will then be soaked with the acetone and present much the same danger as the liquid, with the exception that the acetone will not flow. Disposal of the solventsoaked sorbent and any contaminated soil should also be handled by clean-up professionals.
Acetone, like any other lowflash-point flammable liquid, must not enter any sewer system; the sewer system could fill up with flammable vapors, presenting a city wide explosion hazard. All catch basins and any other entries into the sewer system must be blocked to prevent entry.
Entry into any waterway must also be prevented, and if entry does occur, all downstream users must immediately be notified, especially any industrial users who may take the water into enclosed or heated processes. The acetone will float on the surface of the water as it begins to dissolve. Eventually, all the acetone will either evaporate or dissolve in the water.
If possible, contaminated water should be diverted into a containment area for treatment. Certain materials, such as activated charcoal, may adsorb the acetone and then be removed easily. Aeration, agitation, sparging, and mixing will be helpful in removing the acetone. Again, adsorbent materials holding acetone should be disposed of by experts.
In a fire situation
If the acetone vapors have been ignited, they’ll explode and “flash” back to the spill area. Over large areas, alcohol-type foam will probably be the best attack, blanketing the acetone. It may take a lot of foam to bring the fire under control, and if the fire’s extinguished, vapors may still penetrate the foam and quickly reach the flammable range again. If this occurs, another explosion may occur. In certain special cases, as when there’s no danger to life, exposures, the environment, or systems, it may be wise to allow a controlled burn until the fuel is consumed.
Dry chemical and carbon dioxide may be used as extinguishers where the quantity spilled and other conditions, such as wind and the location of the spill, allow. Here again, one must be concerned with reignition. The energy source for reignition may not be an open flame or spark, but some piece of metal exposed to the fire long enough to be heated above 869° F and thereby reignite vapors being released from the hot liquid.
Water can be used, but it may be ineffective, depending on the mode in which it’s introduced to the fire. Straight streams will just splash the burning liquid around. By contrast, water applied in a fog pattern will cool the fire and disperse and dissolve acetone vapors.
Another extinguishment method uses the dilution principle to give the resulting solution a very high flash point; then the fire will go out.
If the fire is the result of a leak from a container that’s still leaking and which is exposed to the fire, another problem arises. There’s always the danger that flame impingement or radiant heat will cause overpressure in the leaking container. The resulting rapid failure of the container will release large amounts of hot fuel, which will vaporize fast enough to cause an explosion serious enough to kill or injure anyone in the vicinity.
Any closed container exposed to heat may suffer a pressure-relief explosion and, depending on size and shape, it may rocket into the air, trailing burning acetone behind it. Larger containers may or may not leave the ground, but the explosion, dispersal of burning liquid, and rising fireball will produce proportionately larger devastation. The implication here is to try to cool all containers exposed to the heat, including those behind you that may be experiencing the energy of radiant heat.
Protection
As a solvent, acetone softens some rubber and plastic materials, as it penetrates and begins to dissolve them. It won’t attack all polymeric materials, but caution must be used if rubbers and plastics are to be used in containers, hoses, or encapsulating suits.
Protection is relative, depending on the thickness of the polymeric material, the integrity of the seams, the concentration of the chemical, and the duration of contact. Acetone is relatively easy to obtain, and small swatches of the material should be tested with it. Manufacturers claim that suits made from butyl rubber, chlorinated polyethylene, polyethylene, and styrene-butadiene rubber provide the best protection.
Skin contact with acetone over a prolonged period should be avoided, of course, and all contact with the eyes should be prevented. This means all skin should be covered, and the eyes should be protected by splash-proof goggles and face shields.
Positive-pressure, self-contained breathing apparatus should be used when a responder is exposed to high concentrations of acetone. Organic vapor canister masks will work in concentrations up to 5,000 ppm (0.5%).
First aid
For inhalation victims, artificial respiration should be administered, followed by fresh air and immediate medical attention. The victim should be monitored for the onset of delayed symptoms.
For skin contact, affected body areas should be washed with large amounts of soap and water. Medical attention is required for any apparent irritation.
In cases of eye contact, eyes should be flushed with water for 15 minutes, occasionally lifting the eyelids. Medical attention should be immediate.
For ingestion victims, large amounts of water should be administered and vomiting induced, followed by immediate medical attention.


