BY THOMAS O. MURDOCK
People have used a variety of chemicals to commit suicide. Some of the more common chemicals include carbon monoxide, pharmaceutical overdoses, cyanides, inert gases (helium, nitrogen, argon, and carbon dioxide), and pesticides. The use of hydrogen sulfide to commit suicide first appeared in Japan and is now seen with increasing frequency in the United States. The first documented case in the United States was in Pasadena, California, in 2008. Other states that have reported cases of suicide using hydrogen sulfide include Georgia, North Carolina, South Carolina, Indiana, Illinois, Connecticut, Utah, Florida, Pennsylvania, Colorado, Arkansas, Missouri, Massachusetts, South Dakota, and Minnesota.
A good resource for information and emergency response guidance documents related to chemical-assisted suicide incidents is the Colorado State Fire Chiefs’ Association Web page (www.colofirechiefs.org). The International Association of Fire Chiefs Fusion Center has also published emergency response guidelines on its Web site www.hazmatfc.org.
Many technical articles and information briefs have been written documenting the toxicity of hydrogen sulfide, including warning signs at suicide incidents that alert emergency responders to the possibility that it may be present at the suicide scene. However, the initial concentration of hydrogen sulfide in a vehicle and the rate of diffusion (escape) of hydrogen sulfide from the vehicle have not been documented.
Following are the results of experiments conducted by members of the North Metro Chemical Assessment Team that provide information about the initial concentration of hydrogen sulfide and the rate at which the gas concentration decreased when the vehicle doors were opened and the vehicle windows were broken, and through passive diffusion from the closed vehicle. Our data have provided guidelines for emergency responders for safely approaching and mitigating a suicide scene involving hydrogen sulfide.
CHEMISTRY
The reaction of a metal sulfide with a strong acid results in the rapid evolution of hydrogen sulfide gas. Various Internet sources have provided lists of consumer products that contain metal sulfides and strong acids. The most popular metal sulfide used in suicides is calcium sulfide, an active ingredient [28 to 30 weight percent (wt%)] in herbicides that are generically referred to as lime sulfur. Popular sources of acids are toilet bowl cleaners, which usually contain about 20 wt% of hydrochloric acid. These products are supplied in one-quart [947 milliliter (mL)] containers. The balanced equation for the reaction between calcium sulfide and hydrochloric acid is shown below:
Internet suicide instructions suggest adding two to three quarts of lime sulfur to a five-gallon plastic pail, followed by the rapid addition of two to three quarts of products that contain approximately 20 wt% hydrochloric acid. However, note that mixing one quart of lime sulfur [30 wt% calcium sulfide) and one quart of 20 wt% hydrochloric acid would produce approximately 97.5 grams (g) of hydrogen sulfide. The estimated volume of a midsize sedan vehicle is approximately 9 cubic meters (m3); therefore, the estimated concentration of hydrogen sulfide in the vehicle would be 7,772 parts per million (ppm) or 10,833 milligrams (mg)/m3 (assuming uniform mixing in the vehicle). Compare this to the National Institute for Occupational Safety and Health Pocket Guide for Chemical Hazards, which states that a hydrogen sulfide concentration between 1,000 and 2,000 ppm would result in unconsciousness, cessation of respiration, and death in a few minutes.
EXPERIMENTAL DESIGN
One quart (947 mL) of commercially available lime sulfur was placed in a five-gallon plastic pail. The pail containing the lime sulfur was then placed on the front passenger seat in a Ford Escort. A thermocouple was placed in the liquid and attached to a digital thermometer readout. A firefighter in standard structural firefighting clothing and wearing positive-pressure self-contained breathing apparatus (SCBA) rapidly added one quart (947 mL) of toilet bowl cleaner (20% HCI) to the lime sulfur. An immediate reaction ensued, as evidenced by a light yellow foam rising to the top of the five-gallon pail and subsequently overflowing onto the seat of the vehicle within five to 10 seconds. The lime sulfur had a pH of 12; the hydrochloric acid had a pH of 1. Moist pH paper was placed on the front driver and passenger side seat headrests and the steering wheel to determine if the chemical reaction generated a corrosive aerosol.
MONITORING
The temperature of the reaction mixture was measured with a digital thermometer and a thermocouple. Both dry and moist pH paper were used to measure the pH of the liquid and aerosol.
The hydrogen sulfide concentration inside the vehicle was measured with a photoionization detector (PID). Holes were drilled in the roof of the vehicle, and a seven-foot-long flexible tube was connected to the PID and extended into the vehicle to the breathing zone in the driver’s seat (photo 1). A second PID was used to monitor the breathing zone in the passenger side rear seat, and a third PID was used to monitor the floor of the passenger-side rear seat. A four-gas monitor equipped with a hydrogen sulfide sensor was placed on a stand approximately five feet downwind from the passenger door to monitor hydrogen sulfide concentrations outside of the vehicle.
 |
| (1) Car undergoing experiments. (Photo by author.) |
RESULTS AND DISCUSSION
Figure 1 illustrates the rate of increase of hydrogen sulfide concentration inside of the Ford Escort from the reaction of one quart of 28-percent lime sulfur and one quart of 20-percent hydrochloric acid. The concentration of hydrogen sulfide measured at the driver’s breathing zone increased to approximately 8,000 ppm within 30 to 60 seconds after the addition of hydrochloric acid to the calcium sulfide. After the reaction had subsided and the concentration of hydrogen sulfide inside the vehicle was constant (at the 14-minute mark on the graph), all four doors of the vehicle were opened. During a time interval of three minutes, when the doors were open, the concentration of hydrogen sulfide inside the vehicle decreased from 6,000 ppm to less than 5 ppm.
| Figure 1. Hydrogen Sulfide Concentration vs. Time Ventilation by Opening Doors |
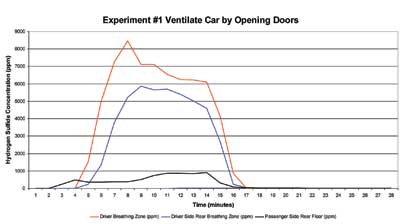 |
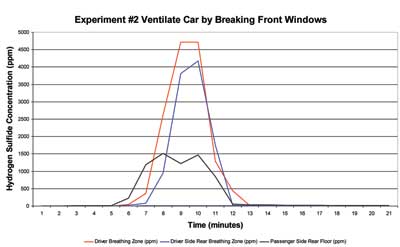
Figure 2 illustrates the rate at which the concentration of hydrogen sulfide decreased when vented from a vehicle by breaking the windows (at approximately the 10-minute mark on the graph) in the driver and passenger doors. During a time interval of three minutes, the concentration of hydrogen sulfide inside the vehicle decreased from 5,000 ppm to less than 5 ppm.
| Figure 2. Hydrogen Sulfide Concentration vs. Time Ventilation by Breaking Windows |
 |
Other studies have shown that the rate of passive diffusion—that is, no broken windows or open doors—of a gas is primarily dependent on the automobile’s volume; the tightness and fit of the doors and windows; whether heating, ventilation, and cooling systems are operating; and pressure differences produced by the speed of the air past the vehicle.1,2
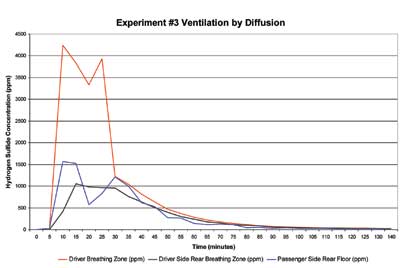
Figure 3 illustrates the rate that the hydrogen sulfide concentration inside the vehicle decreased by passive diffusion. The inside concentration of hydrogen sulfide decreased from 4,000 ppm to approximately 20 ppm from the closed vehicle over a two-hour interval. The final concentration of hydrogen sulfide was between the 10-minute Acute Exposure Guideline Level (AEGL) 1 (0.75 ppm) and AEGL 2 (41 ppm), as published by the U.S. Environmental Protective Agency. The Occupational Safety and Health Administration Permissible Exposure Limit is 20 ppm with a 10-minute maximum at the ceiling. At a concentration of 20 ppm, the odor of hydrogen sulfide will be obvious and unpleasant. Prolonged exposure at this concentration may result in symptoms of dryness and irritation of nose and throat, runny nose, cough, hoarseness, headache, nausea, and shortness of breath. Wear respiratory protection during any operation that involves opening the vehicle. Monitor the air for hydrogen sulfide to determine the risk of exposure to its toxicity.
| Figure 3. Passive Diffusion of Hydrogen Sulfide from a Vehicle |
 |
CONCLUSIONS
- Structural firefighting clothing and SCBA are recommended for any operations that involve opening the vehicle; decrease the level of personal protective equipment only when air monitoring supports the decision.
- The reaction of one quart of 30-percent calcium sulfide and one quart of 20-percent hydrochloric acid in a vehicle resulted in a hydrogen sulfide concentration of 4,000 ppm to 8,000 ppm. These concentrations would certainly be fatal in a few minutes.
- Opening doors or windows for ventilation resulted in a rapid decrease (< 10 minutes) of the hydrogen sulfide concentration.
- Passive diffusion of hydrogen sulfide from a closed vehicle was complete in approximately three hours and is dependent on the volume of the vehicle, the tightness of the fit of the windows and doors, and wind speed.
- The temperature of the reaction mixture increased from ~ 45ºF to 75ºF.
- Hydrogen sulfide will continue to off-gas from porous media (cloth seats and carpeting in the vehicle and the victim’s clothing) after the vehicle is opened.
- No appreciable quantity of aerosol is generated during the acid/base reaction of calcium sulfide and hydrochloric acid based on no change in color of the pH paper.
- Disturbing the container with the reaction mixture results in the generation of more hydrogen sulfide because of the additional mixing.
- The recommended Isolation Perimeter (public exclusion zone) is 50 to 75 yards downwind during vehicle ventilation. This suggested exclusion zone is based on the monitoring results from the four-gas meter near the vehicle and an observation of a firefighter downwind during the time the vehicle was vented. The firefighter noted that the odor of hydrogen sulfide was similar to the odor of mercaptan during a natural gas leak.
References
Englemann R. J., Pendergrass W. R., White J. R., and Hall M. E. (1992). The effectiveness of stationary automobiles as shelters in accidental releases of toxic materials. Atmospheric Environment, 26A, 3119 – 3125.
Park J., Spengler J. D., Yoon D., Dumyahn T., Lee K. and Ozkaynak H. (1998). Measurement of air exchange rate of stationary vehicles and estimation of in-vehicle exposure. J. Exposure Analysis and Env. Epidemiology, 8, 65 – 78.
Thomas O. Murdock earned a Ph.D. in chemistry from the University of North Dakota in 1977, and postdoctoral studies were completed at the Max Plank Institut fur Kohlenforschung (Mulheim, Germany) during 1978 and the University of Minnesota Chemistry Department during 1979-80. He is a retired director of environmental health and safety at the Medtronic World Headquarters Campus in Minneapolis, Minnesota. He is a retired volunteer firefighter and a member of the North Metro Chemical Assessment Team in Anoka County, Minnesota. He has taught numerous courses on hazardous materials chemistry and hazardous materials incident response.
More Fire Engineering Issue Articles
Fire Engineering Archives

