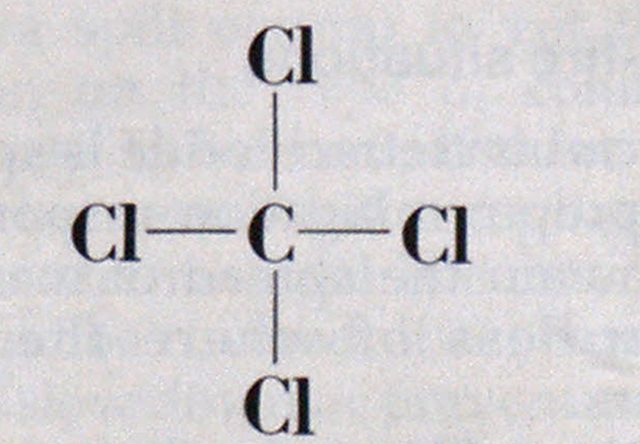
Carbon Tetrachloride
HAZARDOUS MATERIALS
Carbon tetrachloride is a toxic, narcotic, clear, colorless liquid with a sweet, distinctive odor similar to ether. It is probably very familiar to older firefighters, since it was once used very extensively as a fire extinguishing agent. However, hazardous byproducts were produced whenever it was used on a fire or came into contact with hot surfaces, and this brought its use to an end. Carbon tetrachloride is a suspected carcinogen.
The molecular structure of carbon tetrachloride is CC14. Its molecular structure is

Properties
Carbon tetrachloride will not burn (hence its use as a fire extinguishing agent). It has a specific gravity of 1.585, a molecular weight of 153.8, and a vapor density of 5.3. It freezes at — 9.4°F, boils at 170.2°F, and is practically insoluble in water.
It is a stable liquid when water is not present. In the presence of water it will corrode iron and other metals. It does not react with water, but it will react violently with such burning metals as aluminum and magnesium, and with burning wax. It is explosive when heated and in contact with powdered aluminum. It is also reactive with some aluminum compounds, ailyl alcohol, liquid oxygen, organometallic compounds, and several other materials.
Uses
Carbon tetrachloride is used as a solvent for rubber, fats, and oils. It is also used in grain fumigation, metal degreasing, production of semiconductors, and in the manufacture of some plastics, resins, and other chemicals.
Hazards
Carbon tetrachloride is highly toxic by ingestion and inhalation and moderately toxic by skin absorption. Its TLV-TWA (Threshold Limit Value—Time Weighted Average) is 5 ppm, its STEL (Short Term Exposure Limit) is 20 ppm over fifteen minutes, and its IDLH (Immediately Dangerous to Life and Health) limit is 300 ppm. Its odor can be detected at levels as low as 10 ppm. Its decomposition products are highly toxic. Prolonged skin contact with the product may cause defatting of the skin, leading to moderate irritation and dermatitis. Contact with the eyes will produce very painful irritation, redness, and tearing, but not major injury, unless repeated.
Inhalation of the vapors of carbon tetrachloride can cause dizziness, fatigue, weakness, headaches, unconsciousness, and death. Repeated exposure to the vapors can cause liver and kidney damage. Severe damage to heart, liver, and kidneys caused by a delayed reaction several days later has been reported.
Ingestion of carbon tetrachloride can cause irritation of the gastrointestinal tract, nausea, vomiting, diarrhea, and death.
A serious reaction with carbon tetrachloride is that of long-term exposure. Studies have led some agencies to suspect it as being carcinogenic.
Fire situations
Carbon tetrachloride will not burn, but some reference sources hedge by saying that the ignition of carbon tetrachloride is unlikely. Statements like this, plus the listing of carbon dioxide, dry chemical, foam, and water spray as extinguishing agents for carbon tetrachloride, give the impression that, under rather special circumstances, it can burn. This author has no documented cases of it burning, and as stated before, it has been used as a fire extinguishing agent.
However, containers of carbon tetrachloride that are exposed to flames or radiated heat from a fire (or any other source, for that matter) are subject to catastrophic failure due to a buildup of pressure from evaporated material, just like any other liquid. These containers usually are metal drums, and possess no pressure-relief device, so they must be removed from exposure to sources of heat or kept cool by the application of water spray.
Synonyms
benzinoform
carbon chloride
ENT 4,705
flukoids
Halon 104
tetrachloromethane
nectorina
perchloromethane
RCRA Waste Number U211
tetraform
univerm
carbona
carbon tet
fasciolin
Freon 10
methane tetrachloride
tetrafinol
nectorine
R10
tetrachlorocarbon
tetrasol
vermoestricid
Identification Numbers and Ratings
DOT
(Department of Transportation)
ORM-A (Other Regulated Materiai-A)
UN/NA
(United Nations/North America)
1846
CAS
(Chemical Abstract Service)
56-23-5
RTECS
(Registry of Toxic Effects of Chemical Substances)
FG4900000
CHRIS
(Chemical Hazard Response Information System)
CBT
STCC
(Standard Transportation Commodity Code)
4940320
NFPA 704 rating
(National Fire Protection Association)
3-0-0
IMO
(International Maritime Organization)
6.1, poisonous
As with any liquid held in containers, do not get between them and the source of heat unless you can effectively block all the heat. Injuries and deaths have been caused by the sudden explosive rupture of drums that might not even have been noticed by the firefighters. And as with any containers subject to violent failure, water must be applied from as far away as possible.
Any liquid that has been spilled and is exposed to a heat source will evaporate at higher and higher rates as the temperature of the liquid goes up. Carbon tetrachloride is no exception to this rule, and large volumes of harmful vapor will be generated as it absorbs heat. All areas downwind should be evacuated to avoid exposure of the population to these vapors.
Whenever the liquid comes into contact with fire, the fire may be extinguished, but hazardous decomposition products will be generated. Carbon tetrachloride itself is a halogenated hydrocarbon, and its decomposition products will be compounds containing chlorine— predominantly hydrogen chloride. Hydrogen chloride is a corrosive gas that forms hydrochloric acid whenever it dissolves in water, which it does readily. Other chlorinated compounds may be formed, depending on the chemical makeup of the burning material.
There are reports that one of the decomposition products of carbon tetrachloride is the very poisonous gas, phosgene. Respiratory protection must be provided for anyone exposed to the decomposition products of carbon tetrachloride.
Non-fire situations
If carbon tetrachloride is spilled, the proper mitigation response is to contain the spread of material, regardless of where the spill occurs.
If the spill is on land, the spread of carbon tetrachloride may be prevented by the construction of containment ponds. Ponds may be created by using earth-moving equipment to create depressions in the grounds that will hold the liquid. If there is no earth-moving equipment available, the pond may be created by building up earth, sand, clay, and other natural material. Other material, like cement powder, fly ash, or commercial sorbents may be poured around the area to be used as a containment pond.
If the spilled product is not in the area to be used as a containment pond, trenches may be dug to lead the liquid from the spill area to the containment area.
If the proper equipment is available, a pit may be dug to hold the liquid, and trenches dug to lead the liquid to the pit. Containment pits are sometimes preferable to ponds because the surface area of the exposed liquid will be less. This is important for two reasons. First, the rate of evaporation of any liquid is directly proportional to the surface exposed to its environment; therefore, the smaller the area exposed to the atmosphere, the less carbon tetrachloride will evaporate and move downwind. The second reason for the pit’s superiority is that the smaller surface can be covered more easily than the larger area of the pond, thus reducing the amount of material evaporating.
The major disadvantage of using a pit (not counting the time it takes to dig it) is that contamination of the soil may be more extensive and more difficult to remove. Even though more ground surface will be contaminated when a pond is used, the contamination in a pit will be very deep, and the contaminated earth may be difficult to remove. Always consult the proper environmental authorities (who must be notified immediately when a spill occurs) to get their opinion on the type of containment area to be used, if there is a choice between pond and pit.
Once the material is contained, it should be covered, if at all possible, to slow down or prevent evaporation. Rubber or rubber-coated sheets and tarpaulins will not only retard evaporation, they will also keep runoff water or rain water from entering the containment area. Although carbon tetrachloride does not react with water, anv water entering the area will add to its volume, filling the area and perhaps running off. Even though carbon tetrachloride is practically insoluble in water, some small amount is soluble and will spread its contamination as water leaves the containment area.
Regular firefighting foams (nonalcohol-type) are somewhat effective in reducing evaporation when applied to the surface of the liquid, but will not prevent entry of water into the containment area. Foam will also add to the volume of liquid in the pond or pit.
It is important to keep the evaporation rate as low as possible, since carbon tetrachloride’s vapor density of 5.3 is so high that the vapors will flow along low spots (gases and vapors are fluids) for great distances, and will accumulate in low, enclosed areas. The high vapor density also means that the vapors will “hang together” for a great distance, particularly if winds are absent.
Once the liquid has been contained, covering will not be necessary if it is going to be removed immediately. Covering by foam may contaminate the product and make it commercially valueless once it has been salvaged.
If the spill is from a leak rather than from a large failure of the container, recycling the product may be a possibility. If the leaking container can be opened, and the leaking product can be caught in another open container, it might be possible to pump the contained liquid back into the leaking container. This produces a somewhat open system which allows some evaporation, but contamination of the ground is virtually eliminated.
If evaporation is occurring in quantities considered to be hazardous, care must be exercised to contain any runoff created by using high-pressure water fog or spray. No matter how the liquid is contained, the use of high-pressure water fog or spray will “break up” the vapors and disperse them in air more rapidly.
Once the liquid has been contained, the shipper, seller, buyer, or professional salvage operator may suction or pump the material from its containment area to a secure container. Salvage is the job of professionals who are properly educated, trained, and equipped to handle such operations. Fire departments should not involve themselves in salvage procedures unless it is an emergency and they are similarly educated, trained, and equipped.
For small spills of carbon tetrachloride or for the residue after pumping, the material can be absorbed by the use of cement powder, fly ash, or other commercial sorbent material. Soil and clay may also be used. Once the liquid has been absorbed, it can be removed by professionals and disposed of properly. Absorbed material is still the same material it was before absorption; the absorbed carbon tetrachloride, therefore, will still be hazardous.
Once the product has been removed, the proper environmental authorities will decide how much soil and other materials will have to be removed to decontaminate the area. This soil removal should be done by professionals who will dispose of the contaminated soil according to federal, state, and local regulations.
All efforts must be expended to keep carbon tetrachloride from reaching streams and waterways. Catch basins should be diked to prevent the liquid from entering sewers. Sewer pipes will quickly fill with vapors, and sewage treatment facilities should be warned immediately.
If carbon tetrachloride enters a stream or waterway, it will sink to the bottom due to its near insolubility in water and its specific gravity of 1.58. Some small amount of product will dissolve.
If at all possible, the waterway should be diverted into low-lying ground that can contain the contaminated water. Once the water has slowed or stopped moving, the carbon tetrachloride will consolidate at the lowest spot, and at that point can then be removed by suction.
Some authorities recommend excavating lagoons within the waterway to encourage the carbon tetrachloride to accumulate in such created low spots, but the environmental authorities may object. Natural low spots in the waterway bed might allow the product to gather there, if the movement of water is sufficiently slow. Any rapidly moving water will break up the product into small sphere-like particles and make them difficult to trap. If the product can be made to accumulate in low spots, it may be suctioned out by use of the proper equipment and pumped into secure containers. Dredging may also be used, but may be prohibited by environmental authorities. If these techniques are permitted by the authorities and feasible, all pumped or dredged material must be disposed of properly.
In all cases, whether the material may be contained or not, all downstream users of the water must be notified immediately.
The presence of other chemicals in the water and/or the presence of rapids or waterfalls on the waterway might cause the carbon tetrachloride to be emulsified, and thus not be recoverable.
Water that has been contained and has had the insoluble liquid removed may be decontaminated by aeration, air stripping, or sparging. Aeration may be accomplished by spraying the water into the air, while air stripping and sparging involve bubbling air or an inert gas through the water. Any of these techniques will cause the carbon tetrachloride vapors to enter the air near the area where the purifying procedures are occurring.
Some spilled product may be removed from the water by the addition of activated charcoal into the water followed by a mixing action. The charcoal removed from the water with carbon tetrachloride adsorbed must be disposed of properly.
Protective equipment
To keep the material from contacting the skin, rubber boots and gloves, in addition to full face shields and turnout clothing that is impervious to the liquid, must be worn. Materials that will protect include nitrile rubber, nitrile-butadiene rubber, polyurethane, and Viton. For respiratory protection, positive-pressure, self-contained breathing apparatus must be used.
First aid
If carbon tetrachloride has been inhaled, move the victim to fresh air. Artificial respiration must be administered if breathing is difficult or has stopped. (Warning: Mouth-to-mouth resuscitation may expose the responder to the chemical in the victim’s lungs or vomit.) Get medical attention immediately. Resources warn against the use of epinephrine or ephedrine because of possible effects on the heart.
If the product has been ingested and if the victim is conscious, give large amounts of water or a strong solution of salt water and induce vomiting. If the victim is unconscious, do not try to administer water or try to induce vomiting. Get medical attention immediatefy-
If contact has been made with the skin, remove contaminated clothing, being sure to protect against contact. Wash affected areas with soap and water. Get immediate medical attention. Defatting of the skin may occur.
If contact with the eyes occurs, flush immediately with water for at least 15 minutes, occasionally lifting the eyelids. Get immediate medical attention.
Glossary
Absorption—The penetration of one substance into the inner structure of another substance.
Adsorption—The adherence of one material on the surface of another.
Aeration—The process by which air is passed through a liquid.
Air stripping—A process by which a material is pumped through an air chamber or sprayed into the open air at high pressure in order to remove contaminants.
Carcinogen—A substance that causes cancer.
Chlorinated—The incorporation of chlorine into a chemical compound.
Emulsified—The condition of two or more immiscible liquids held in suspension with each other.
Halogenated—The incorporation of a halogen (fluorine, chlorine, bromine, or iodine) into a chemical compound.
Hydrocarbon—A chemical compound containing only carbon and hydrogen.
ORM-A—A U.S. Department of Transportation designation for “Other Related Material.” The “A” stands for materials that have an anesthetic, irritating, noxious, toxic, or other similar property, that can cause an extreme annoyance or discomfort to passenger or crew if a leak occurs during transportation.
Sparging—The act of bubbling air or another gas through another medium.

